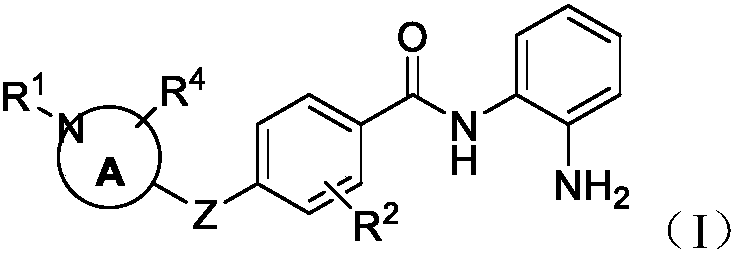
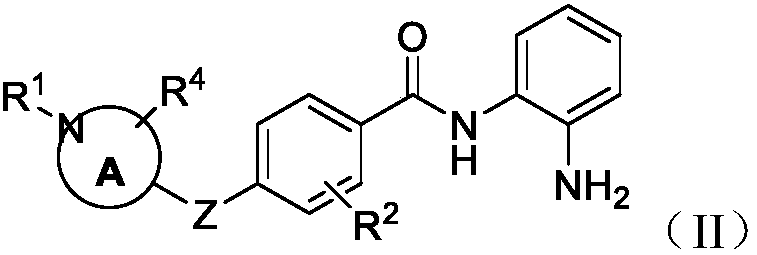
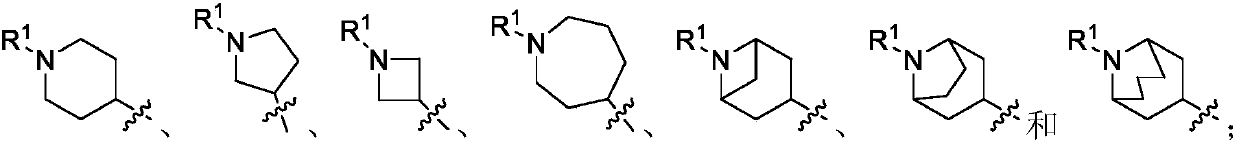
Histone deacetylase inhibtors
A compound, cycloalkyl technology, applied in anti-inflammatory agents, drug combinations, organic chemistry, etc., can solve the problem of little understanding of the role of specific isoforms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0140] General procedure for compounds 1-6: General procedure for Boc deprotection : To a stirred solution of tert-butoxycarbonyl (Boc)-protected compound (1 eq) in dioxane:methanol (4:1, 5 vol) was added 4N HCl in dioxane (3 vol) and heated at room temperature The reaction mixture was stirred under pressure. Reaction progress was monitored by TLC. After completion, the reaction mixture was evaporated to dryness (if the compound precipitated, it was then filtered and further purified). The crude product was purified by trituration with ether / pentane / MTBE or by preparative HPLC to afford the title compound.
[0141] General procedure for N-alkylation : Procedure A: To a stirred solution of the amine substrate (1 eq) and cesium carbonate / potassium carbonate (3 eq) in DMF (10 vol) was added the corresponding alkyl halide (1.1 eq). The reaction mixture was heated at 80 °C for 5h to 30h. Reaction progress was monitored by TLC. After completion, the reaction mixture was pour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com