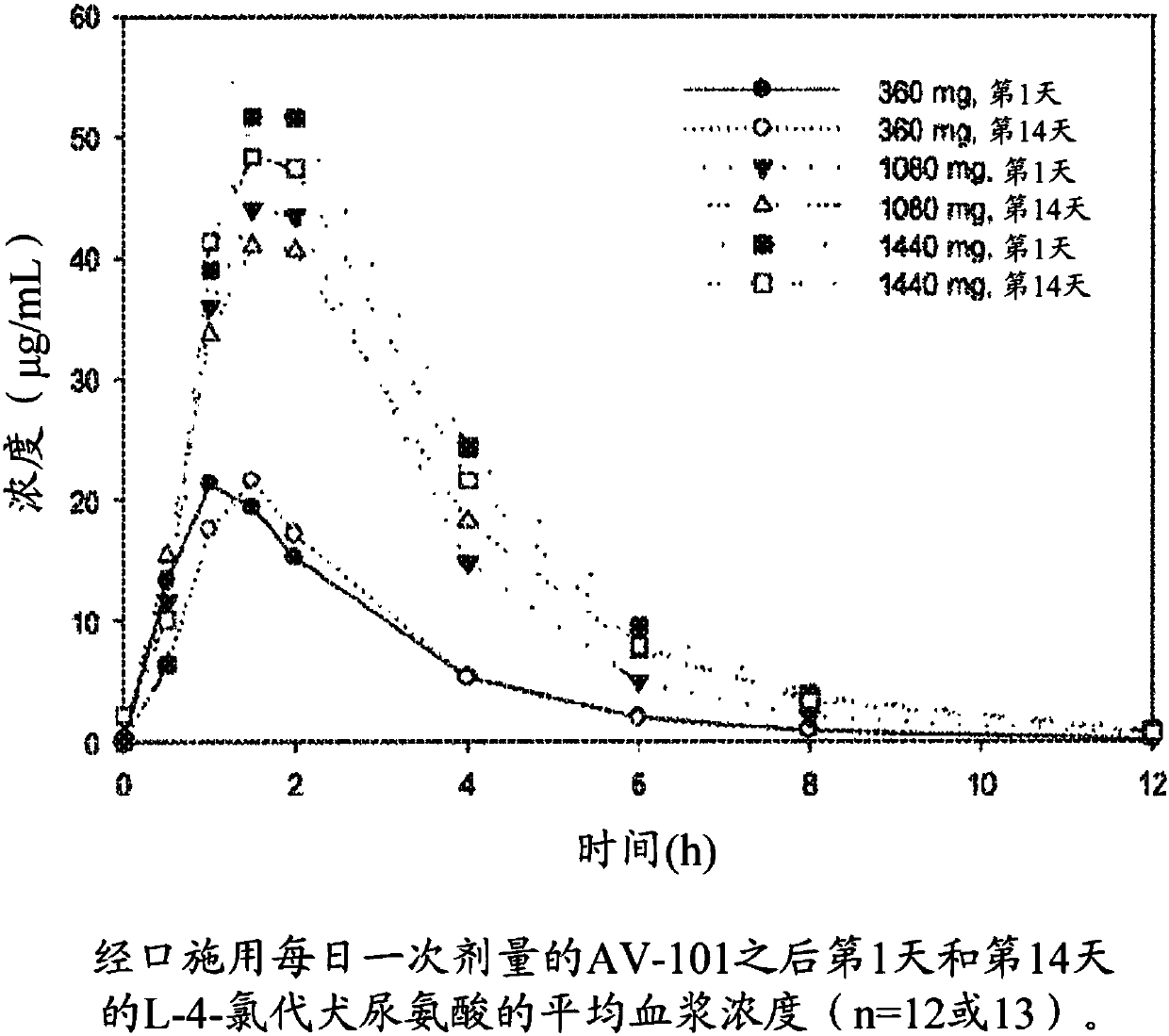
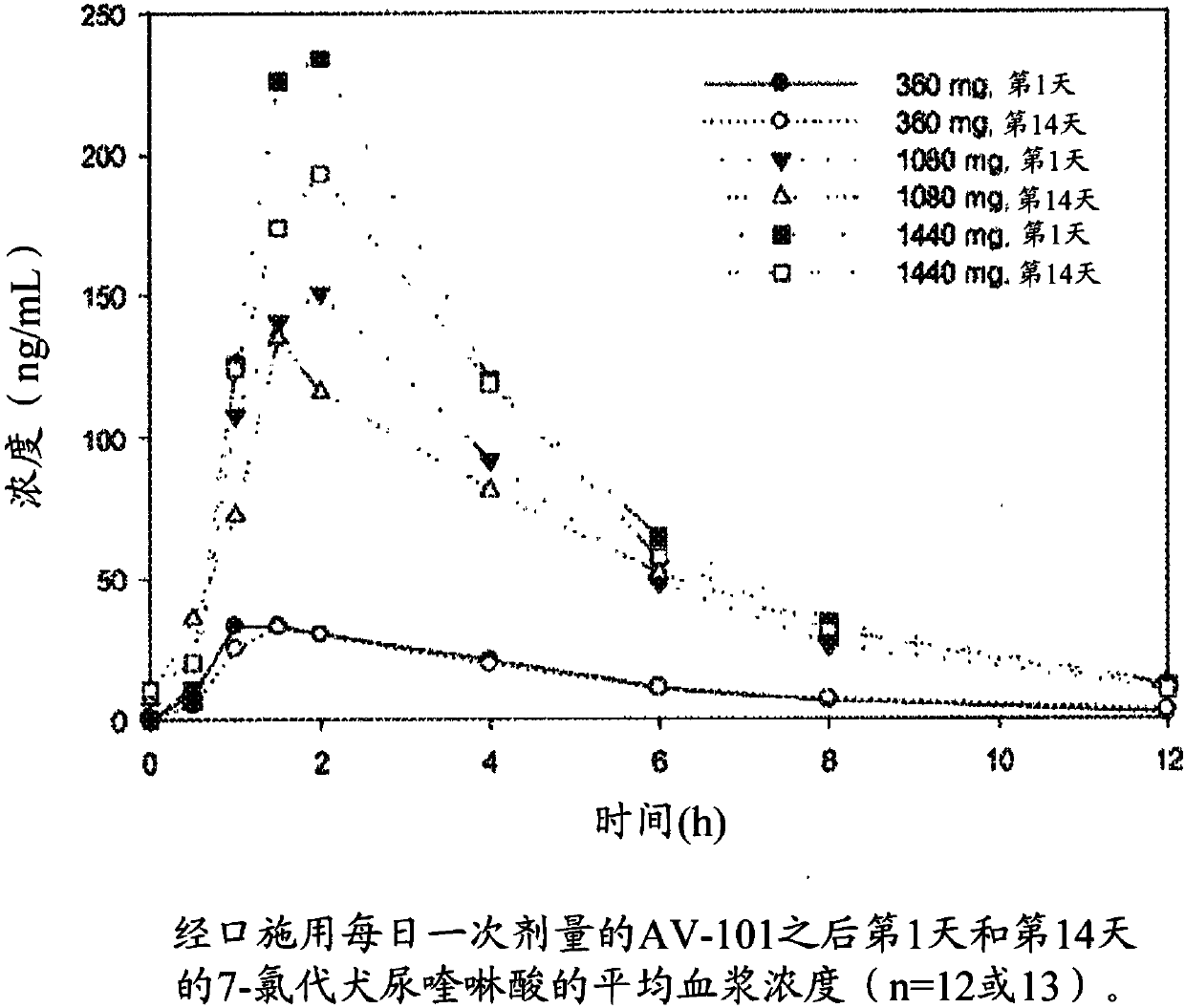
Therapeutic uses of l-4-chlorokynurenine
A technology of kynurenine and chlorination, which is applied to medical preparations containing active ingredients, metabolic diseases, pharmaceutical formulas, etc., and can solve problems such as limiting therapeutic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
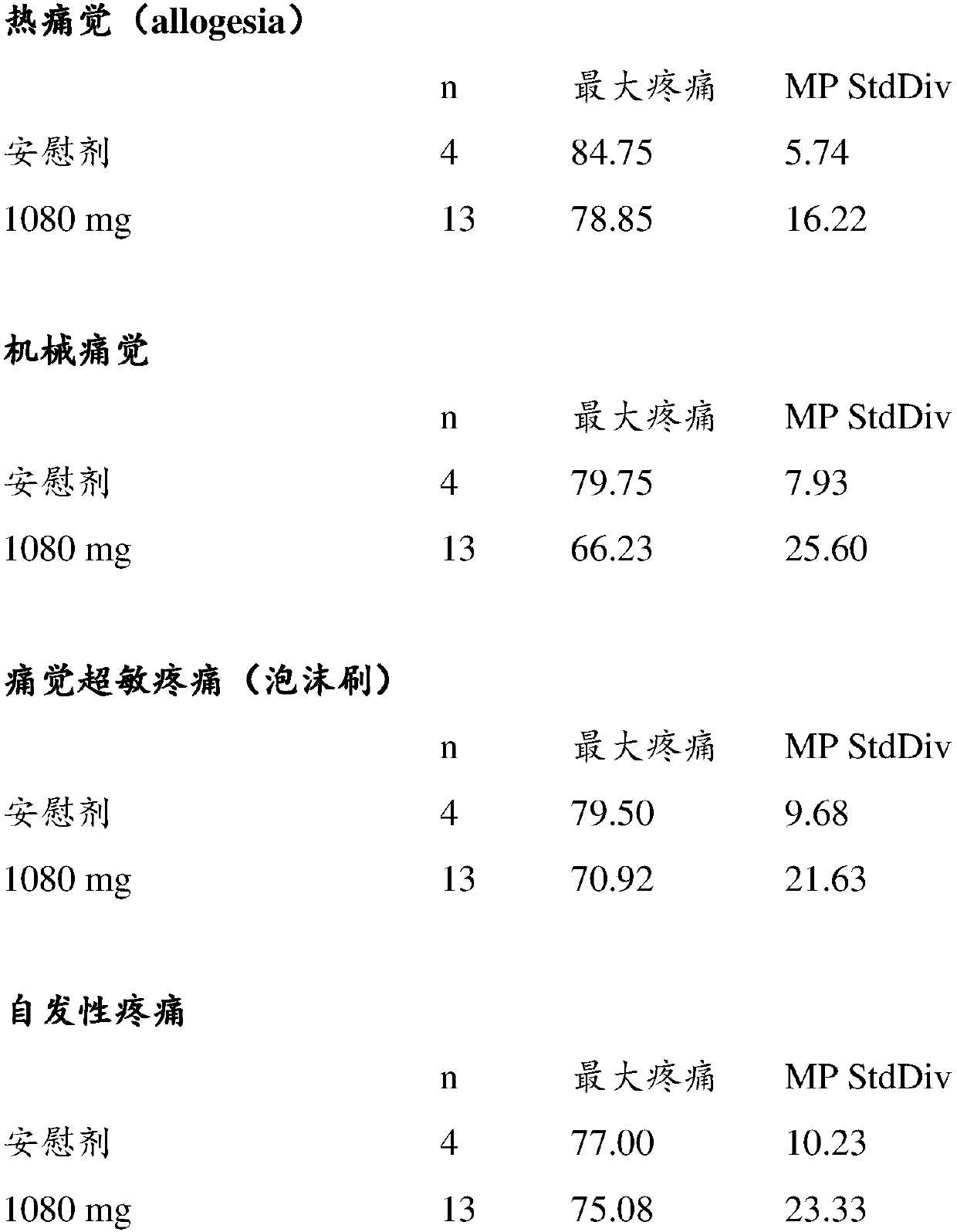
[0053] Example 1: Antihyperalgesic effect of L-4-chlorokynurenine on capsaicin-induced hyperalgesia.
[0054] On days 1 and 14 of the clinical study, two sequential intradermal injections of 250 μg capsaicin were injected into the volar side of the alternating forearm to produce burning pain, secondary hyperalgesia and flare. Capsaicin USP (United States Pharmacopoeia) was prepared according to standard procedures in the field and dissolved in 20% cyclodextrin at a concentration of 10 mg / mL.
[0055] The first capsaicin injection was administered in one forearm 1 hour after oral administration of L-4-chlorokynurenine or placebo and 2 hours after administration of L-4-chlorokynurenine or placebo A second capsaicin injection was given in the other forearm. Sensory nerve testing begins immediately with each capsaicin injection. Continuous pain was assessed using a 100-mm visual analog scale (VAS) before injection and at 0, 5, 10, 15, 30, 45, and 60 min after each capsaicin inje...
Embodiment 2
[0068] Example 2: Antidepressant activity of L-4-chlorokynurenine
[0069]Surprisingly, the inventors also discovered mood enhancing or antidepressant activity of L-4-chlorokynurenine. In the clinical study described in this application, 5 of 26 subjects (compared to zero subjects in the placebo group) reported affirmative feelings of well-being. This is consistent with reports that the glutamatergic system contributes to the pathophysiology of depression and that stress can induce changes in NMDA receptors. See, eg, Calabrese et al. 2012.
Embodiment 3
[0070] Example 3: Treatment of Major Depressive Disorder (MDD)
[0071] Twenty-five male and female patients aged 18 to 65 diagnosed with MDD were treated with L-4-chlorokynurenine (1080 mg / day or 1440 mg / day orally) for two weeks, similar in design to In similar studies [Ibrahim et al 2012, Zarate et al 2013, Zarate et al 2006, Zarate et al 2005]. Improvement in overall depressive symptoms was measured by either the Hamilton Depression Rating Scale (HDRS) [Hamilton 1959] and the Montgomery Asberg Depression Rating Scale (MADRS) total score or Significant reductions in both are shown [Montgomery et al 1979]. For a given patient, an additional measure of treatment efficacy may also be the proportion of subjects achieving remission (HDRS ≤ 7) and response (≥ 50% reduction in HDRS total score from baseline); changes from baseline in the following measures: Hamilton Anxiety The Hamilton Anxiety Rating Scale (HAM-A) [Hamilton 1959], the Columbia Suicide Severity Rating Scale (C-S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com