A diagnostic marker for bladder cancer and its application and diagnostic kit
A technology for diagnosing marker and bladder cancer, applied in the field of biomedicine, can solve the problems of invasive cost of cystoscopy, easy missed diagnosis of bladder carcinoma in situ, misdiagnosis, low detection sensitivity, etc., and achieve good diagnostic efficiency and diagnostic results. High and accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Screening for diagnostic markers:
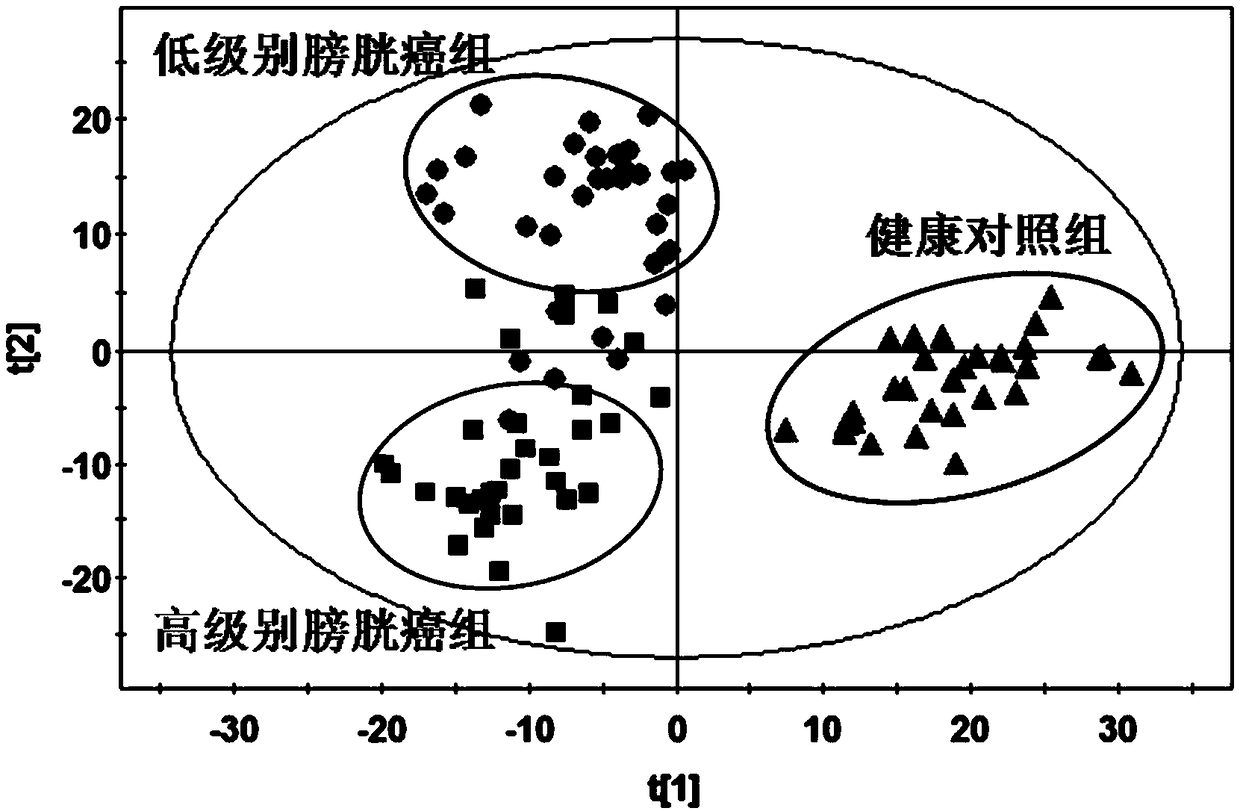
[0031] (1) Collection of serum samples: 34 serum samples from low-grade and high-grade bladder cancer patients were collected as the low-grade bladder cancer group and high-grade bladder cancer group, and 30 serum samples from healthy controls were used as the healthy control group. Samples were stored frozen at -80°C;
[0032] (2) Serum sample pretreatment: take 100 μL of serum sample after freezing and thawing, add 300 μL methanol (containing 12.5 μg / ml L-2-chloropropanine, used as internal standard) vortex for 1 min, and then place in ice water bath for ultrasound Extract for 10 minutes, centrifuge at 14000g for 15 minutes at 4°C, absorb the supernatant and inject the sample;
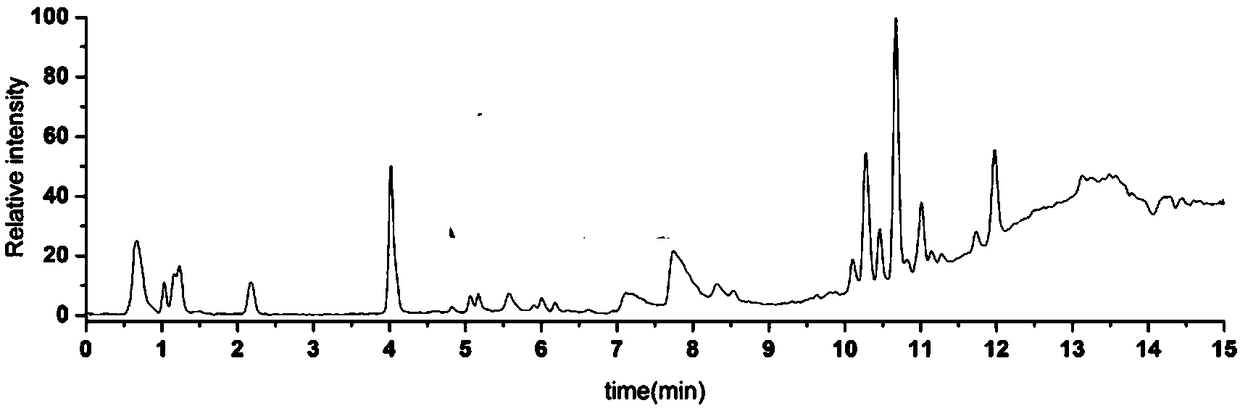
[0033] (3) Sample liquid chromatography-mass spectrometry analysis: Serum samples were analyzed by ultra-high performance liquid chromatography and mass spectrometry. The liquid chromatography was an Agilent 1290 Infinity liquid chromatography system with a...
Embodiment 2
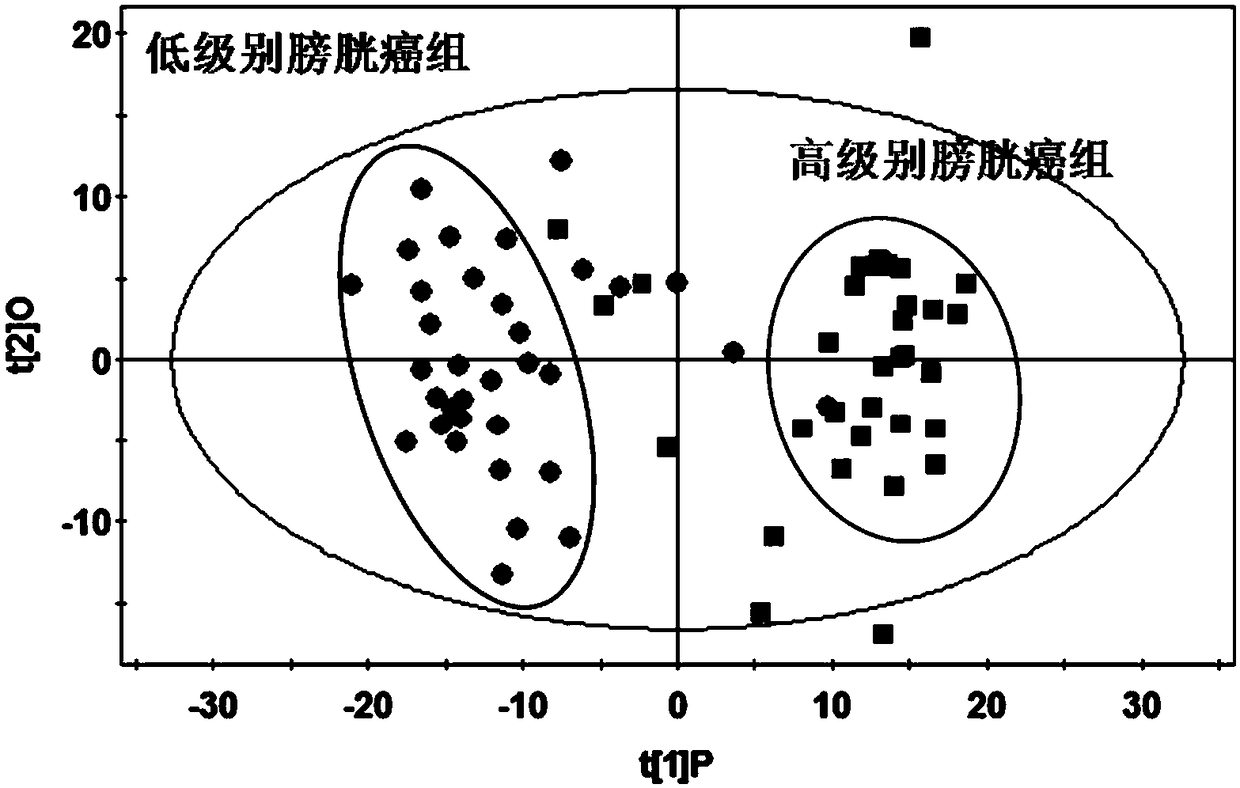
[0044] Validation of the diagnostic efficacy of high-grade bladder cancer from low-grade bladder cancer:
[0045] In order to quantitatively evaluate the diagnostic efficacy of the model for high-grade bladder cancer and low-grade bladder cancer, a receiver operating curve (ROC curve, Figure 7 ). The results of the ROC curve in this study showed that the area under the ROC curve calculated from the quantitative data of the three diagnostic markers in 34 cases of high-grade bladder cancer and 34 cases of low-grade bladder cancer reached 0.961, the sensitivity reached 88.2%, and the specificity Up to 91.2%, these results indicate that the combination of these three diagnostic markers has a high accuracy in distinguishing high-grade bladder cancer from low-grade bladder cancer. The distribution of P values is shown in Figure 8 As shown, when reaching the highest sensitivity (88.2%) and specificity (91.2%), the optimal cut-off value of model P is equal to 0.4669, is cut-off ...
Embodiment 3
[0047] Diagnostic kits and diagnostic kits in the diagnosis and differentiation of high-grade bladder cancer and low-grade bladder cancer:
[0048] The diagnostic kit of the present embodiment includes a diagnostic reagent and a diagnostic model, and the diagnostic reagent includes inosine, N-acetyl-N-2-formyl-5-methoxykynurenine and phosphatidylserine (O-18: 0 / 0:0) standard products of the three markers, the diagnostic model is:
[0049]
[0050] Among them, W 肌苷 Represents the peak area of inosine in serum samples detected by liquid chromatography-mass spectrometry, W 犬尿氨酸 Representative peak area of N-acetyl-N-2-formyl-5-methoxykynurenine in serum samples detected by liquid chromatography-mass spectrometry, W 磷脂酰丝氨酸 Represents the peak area of phosphatidylserine (O-18:0 / 0:0) in serum samples detected by liquid chromatography-mass spectrometry, W 内标 Represents the peak area of the internal standard L-2-chlorophenylalanine in the serum samples detected by liquid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com