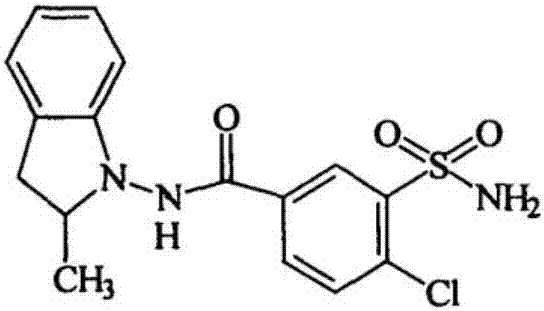
Stable storage indapamide sustained release tablet and preparing process thereof
A technology of indapamide and sustained-release tablets, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The composition is as follows in Table 1:
[0025] Table 1
[0026] Raw materials
Batch: 1000 pieces
1.5g
124.5g
Hypromellose 4000cps
72g
Micropowder silica gel
1g
1g
[0027] Preparation:
[0028] (1) Initial mixing: the prescription amount of indapamide and lactose mixed;
[0029] (2) Sieve: sieve the above-mentioned mixed material with 30 meshes;
[0030] (3) Mixing: after sieving, the material is evenly mixed with hypromellose 4000cps;
[0031] (4) final mixing: the above-mentioned mixed material is mixed with micropowder silica gel and magnesium stearate;
[0032] (5) Tabletting: The above materials are pressed into tablets with a punching die of No. Φ8.
Embodiment 2
[0034] The composition is as shown in Table 2:
[0035] Table 2
[0036] Raw materials
Batch: 1000 pieces
Indapamide
1.5g
123.5g
Hypromellose 4000cps
72g
hydrogenated castor oil
3g
[0037] Preparation:
[0038] (1) initial mixing: the indapamide and microcrystalline cellulose of prescription quantity are mixed;
[0039] (2) Sieve: 24 meshes of above-mentioned mixed materials are sieved;
[0040] (3) Mixing: after sieving, the material is evenly mixed with hypromellose 4000cps;
[0041] (4) final mixing: above-mentioned mixed material is mixed with hydrogenated castor oil;
[0042] (5) Tabletting: The above materials are pressed into tablets with a punching die of No. Φ8.
Embodiment 3
[0044] The composition is as follows in Table 3:
[0045] table 3
[0046] Raw materials
Batch: 1000 pieces
Indapamide
1.5g
127.5g
68g
talcum powder
1g
hydrogenated castor oil
2g
[0047] Preparation:
[0048] (1) Initial mixing: the prescription amount of indapamide and lactose mixed;
[0049] (2) Sieve: sieve the above-mentioned mixed material with 30 meshes;
[0050] (3) Mixing: after sieving, the material is evenly mixed with hydroxypropyl cellulose HXF;
[0051] (4) Final mixing: the above-mentioned mixed material is mixed with talcum powder and hydrogenated castor oil; (5) Tabletting: the above-mentioned material is pressed into a tablet with a die of Φ8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com