Cetirizine injection
A technology of cetirizine and injection, which is applied in the field of pharmaceutical preparations and can solve problems such as unstable properties, no hemolysis of parent hydroxyzine, and impact on product safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
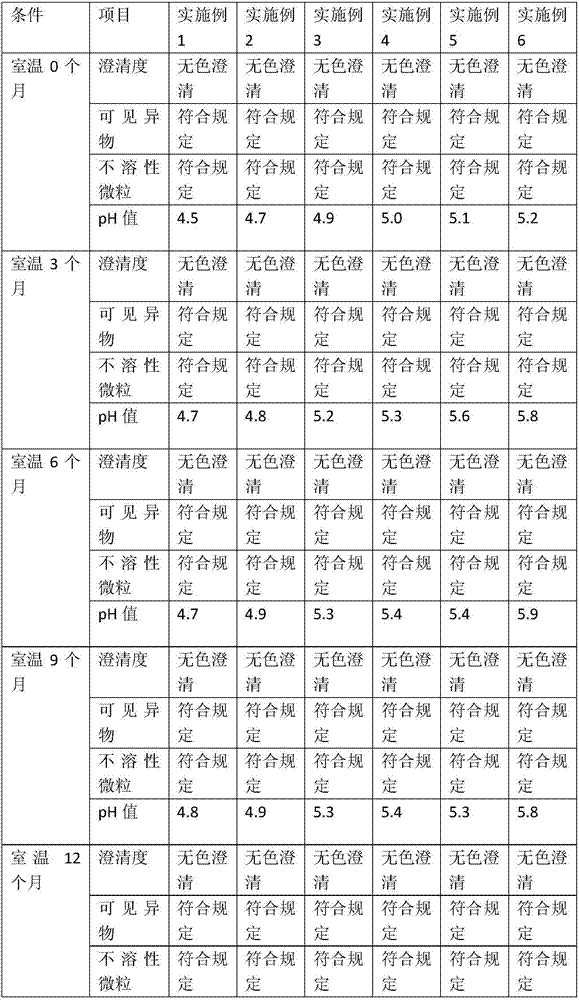
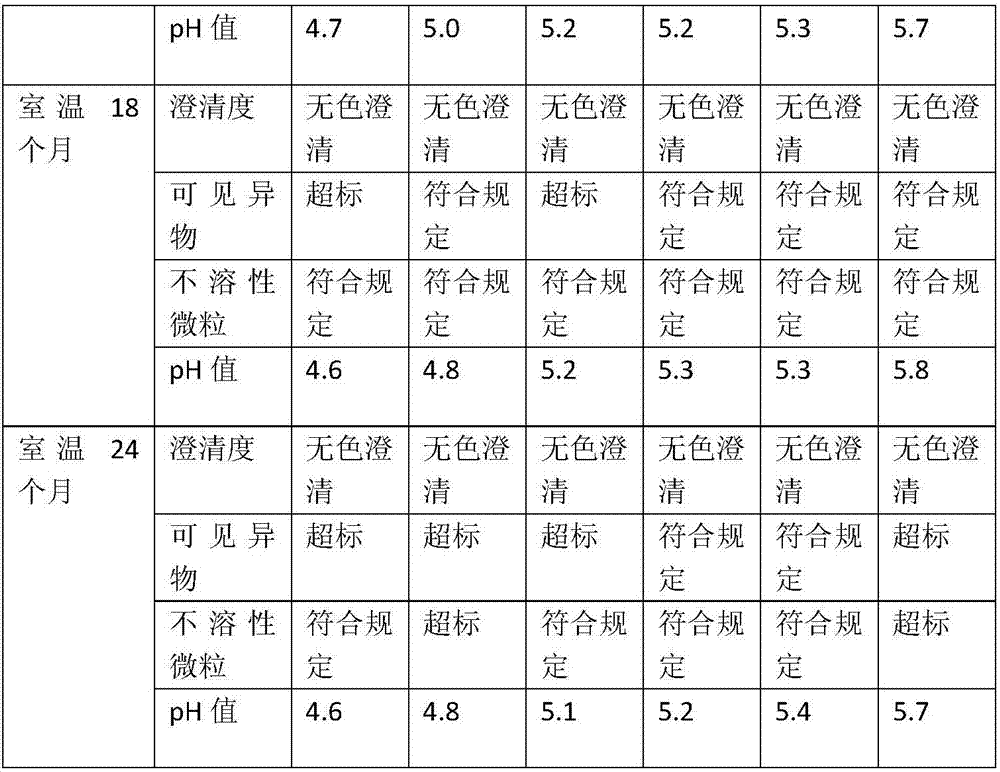
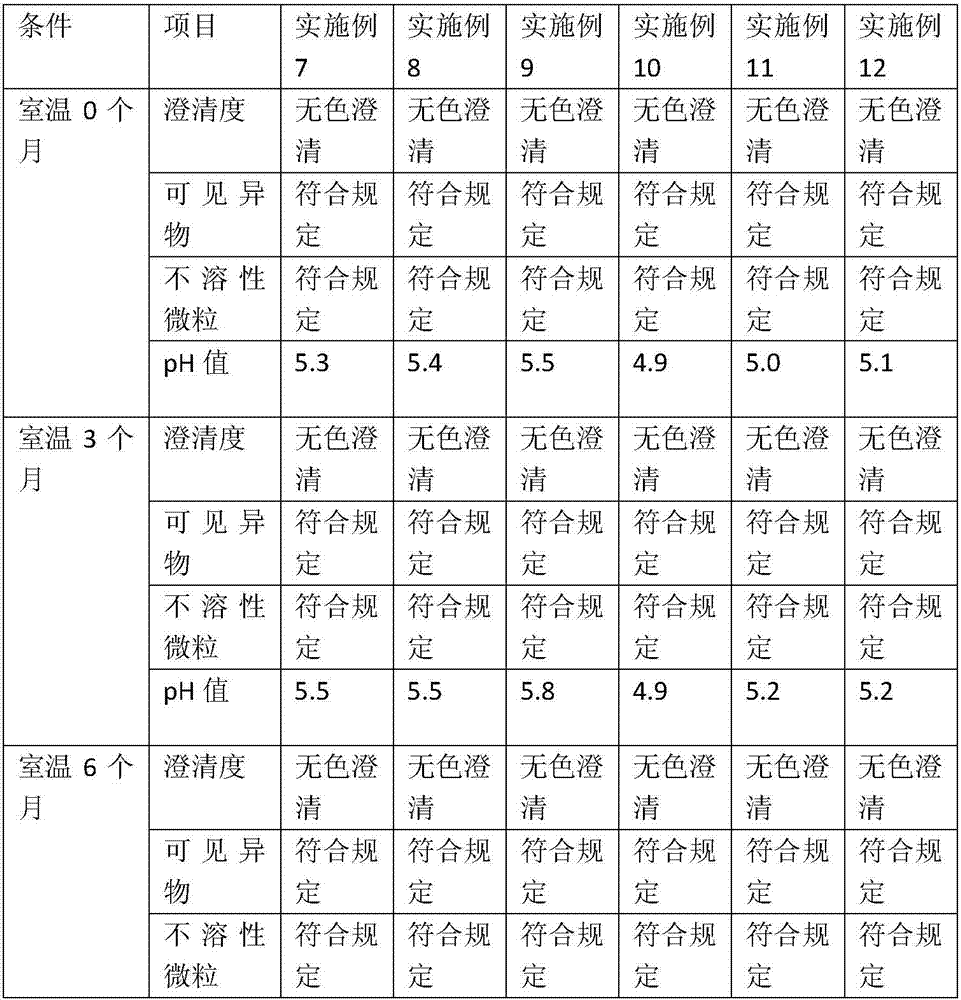
Embodiment 1
[0027] Single bottle dose: total volume: 1ml / bottle, cetirizine volume: 10mg / bottle, the prescription is as follows:
[0028] Cetirizine Hydrochloride 10mg
[0030] pH adjusted to 4.5
[0031] Appropriate amount of water for injection
Embodiment 2
[0033] Single bottle dose: total volume: 1ml / bottle, cetirizine volume: 10mg / bottle, the prescription is as follows:
[0034] Cetirizine Hydrochloride 10mg
[0036] pH adjusted to 4.7
[0037] Appropriate amount of water for injection
Embodiment 3
[0039] Single bottle dose: total volume: 1ml / bottle, cetirizine volume: 10mg / bottle, the prescription is as follows:
[0040] Cetirizine Hydrochloride 10mg
[0042] pH adjusted to 4.9
[0043] Appropriate amount of water for injection
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com