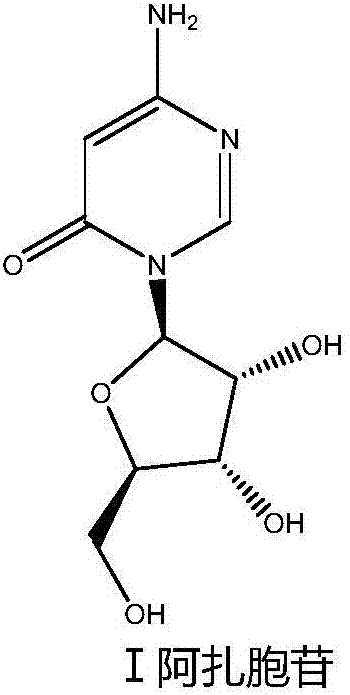
Azacitidine preparation and method for preparing same
A technology of azacitidine and azacitidine, which is applied in the field of preparation of azacitidine and its preparation, can solve the problems of increased product risk, increased impurities and high cost of packaging materials, avoids changes in appearance and properties, and saves packaging materials cost, the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] prescription
[0030]
[0031] Preparation Process:
[0032] Weigh the prescribed amount of sodium pyrosulfite and azacitidine, add 70% of the prepared volume of water for injection, stir and mix evenly, adjust the pH of the medicinal solution with a pH regulator, and control the pH of the medicinal solution to 6.2 after the main ingredients are completely dissolved. Add the prescribed amount of glycerin and poloxamer 188, stir and mix evenly, add water for injection to the total amount of preparation, adjust the pH of the liquid medicine with a pH regulator, and control the pH value of the liquid medicine to 6.0 after the main drug is completely dissolved, stir, Filtration, filling, fusion sealing, light inspection, and packaging.
Embodiment 2
[0034] prescription
[0035]
[0036] Preparation Process:
[0037] Weigh the prescription amount, sodium pyrosulfite and azacitidine, add 60% of the prepared volume to the water for injection, stir and mix evenly, adjust the pH of the medicinal solution with a pH regulator, and control the pH of the medicinal solution to 5.6 after the main drug is completely dissolved. , add the prescribed amount of glycerin and poloxamer 188, stir and mix evenly, add water for injection to the total amount of preparation, adjust the pH of the liquid medicine with a pH regulator, and control the pH value of the liquid medicine to 5.5 after the main drug is completely dissolved, and stir , filtered, filled, melt-sealed, light inspected, and packaged.
Embodiment 3
[0039] prescription
[0040]
[0041] Preparation Process:
[0042]Weigh the prescription amount, sodium pyrosulfite and azacitidine, add 80% of the prepared volume to the water for injection, stir and mix evenly, adjust the pH of the medicinal solution with a pH regulator, and control the pH of the medicinal solution to 6.7 after the main drug is completely dissolved , add the prescribed amount of glycerin and poloxamer 188, stir and mix evenly, add water for injection to the total amount of preparation, adjust the pH of the liquid medicine with a pH regulator, and control the pH value of the liquid medicine to 6.5 after the main drug is completely dissolved, and stir , filtered, filled, melt-sealed, light inspected, and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com