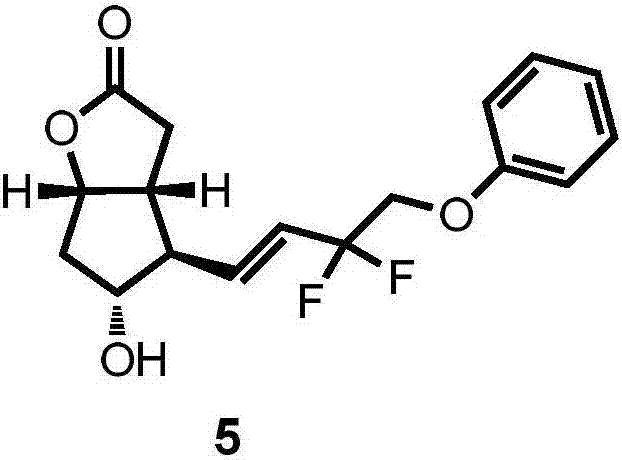
Preparation method for tafluprost intermediate
A technology of reaction time and compound, applied in the field of pharmacy, can solve problems such as unclear mechanism of action, and achieve the effect of enhancing crystallization performance, reducing reactivity and improving crystallization performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
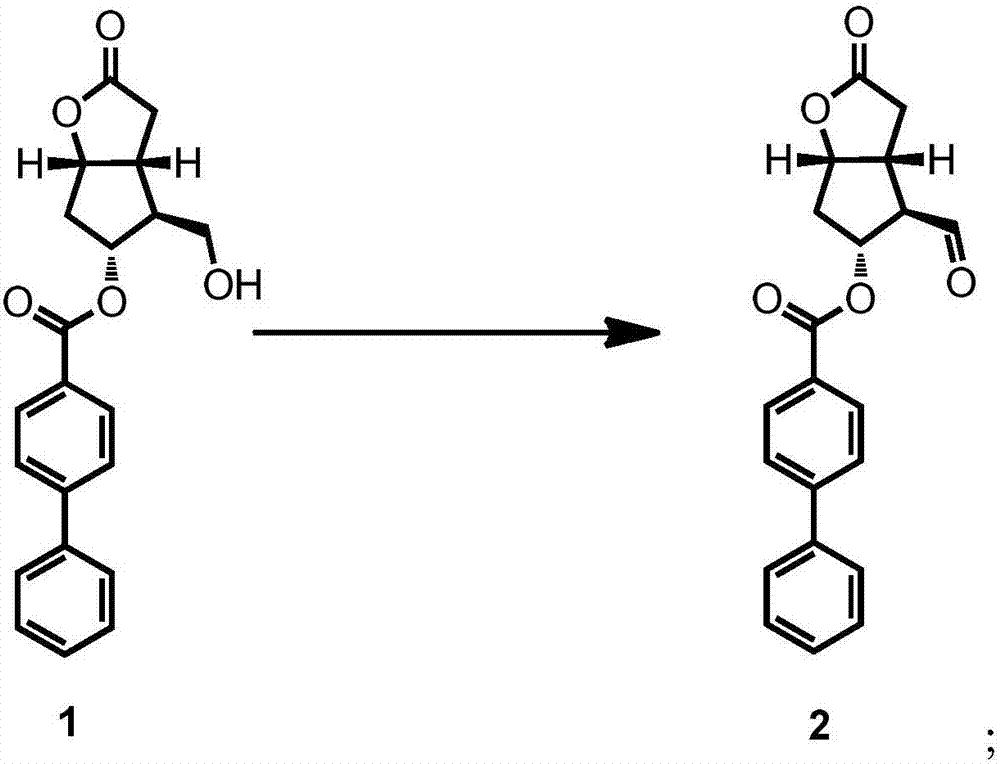
[0044] The preparation of formula 2 compound
[0045] Dess-Martin reagent (86.66g, 1.2eq) was added to a 2L three-necked flask containing DCM (480mL) at one time, and mechanical stirring was started for 10 minutes to obtain a white turbid solution. The temperature was cooled in an ice-water bath for 30 minutes, and at 22° C., a solution of the compound of formula 1 (60.00 g) in DCM (240 mL) was added dropwise into the three-necked flask within about 30 minutes. After the middle stage of the dropwise addition, part of the solution is clear, and the turbidity slowly increases in the later stage. After dropping, the temperature was raised naturally and stirred for 12 hours. Cool down to 0-5°C in an ice-water bath, add 600mL of DCM to dilute; drop in the prepared Na 2 S 2 o 3 Aqueous solution (from Na 2 S 2 o 3 161.5g, 2.6eq and 600mL of water), after the dropwise addition, keep stirring for 2h until the system is clear. Separate the liquids, combine the organic phases, co...
Embodiment 2
[0047] The preparation of formula 2 compound
[0048] Anelli oxidation reagent consisting of NaOCl oxidant (15.2 g, 1.2 eq), Tempo catalyst (2.6 g, 0.1 eq) and NaBr co-oxidant (20 g, 1.0 eq) was added in one portion to a container containing DCM (400 mL) and isopropanol ( 80ml) in a 2L three-neck flask, start mechanical stirring, and stir for 10 minutes. The temperature was cooled in an ice-salt bath for 30 minutes, and at -5-0° C., a solution of the compound of formula 1 (60.00 g) in DCM (240 mL) was added dropwise into the three-necked flask within about 30 minutes. After the mid-term of the dropwise addition, part of it was dissolved. The turbidity increases slowly in the later stage. After dropping, keep stirring at -5-0°C for 1h. Cool down to -5-0°C in an ice-water bath, add 600mL of DCM to dilute; drop in the prepared Na 2 S 2 o 3 Aqueous solution (from Na 2 S 2 o 3 161.5g, 2.6eq and 600mL of water), after the dropwise addition, keep stirring for 2h until the sy...
Embodiment 3
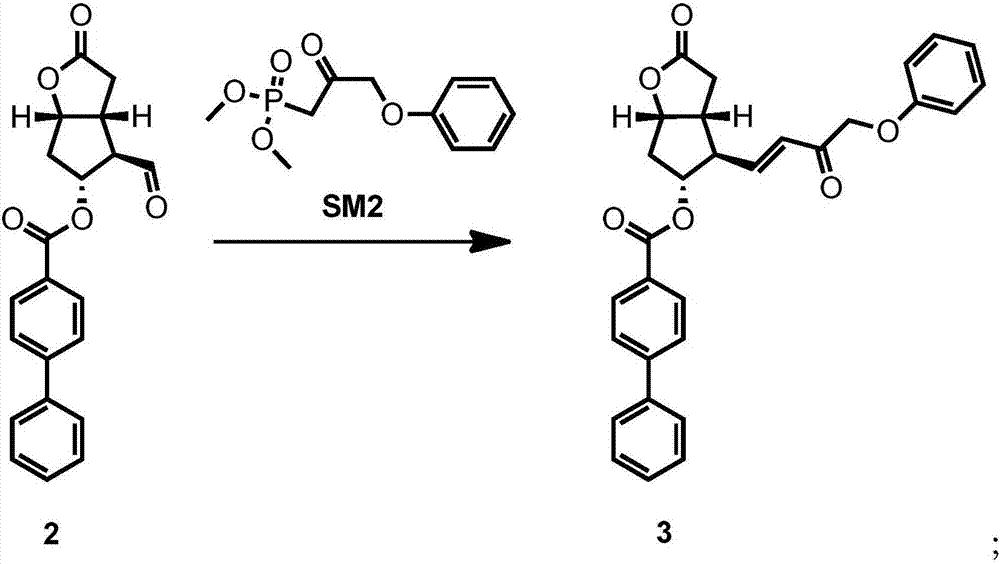
[0050] The preparation of formula 3 compound
[0051] At 5° C., a solution previously prepared from LiOH (1.44 g, 1.2 eq) and water (12 mL) was added dropwise to a solution of HWE reagent (1.44 eq) in DCM (120 mL). After stirring and activating for 30 min, the solution of the compound of formula 2 prepared in Example 1 was started to be added dropwise at 5°C. After the dropwise addition was completed, it was raised to 22°C naturally and kept stirring for 19h. At 22°C, start adding citric acid aqueous solution (20 mL, 0.5 mole / L) dropwise to quench the reaction, and adjust the pH of the water phase to 1-2. After 10 minutes of dripping, the clarity of the water phase increases. Stirring was maintained for 1 h. The phases were separated, the aqueous phase was extracted with DCM, and the organic phases were combined. Add 200 mL of absolute ethanol to the organic phase, spin off the low-boiling solvent DCM, and some white solids are precipitated. Add absolute ethanol until the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com