Biomedical application of ophiobollin to preparation of Gli gene inhibitor and liver cancer prevention and treatment drug
A technology of staurosporine and biomedicine, which is applied in the direction of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc., and can solve the problem of confidentiality and other issues.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
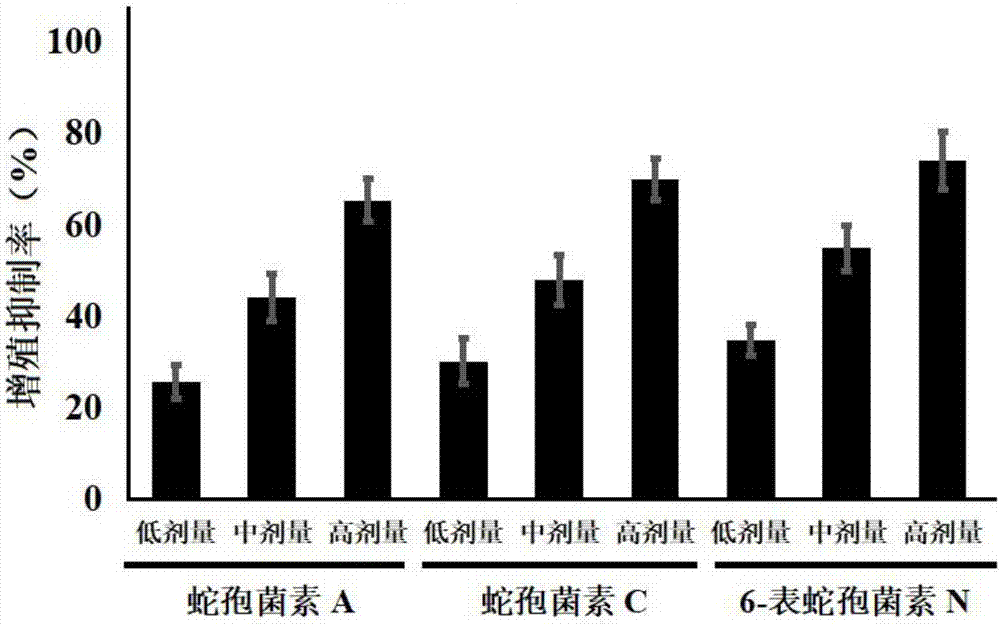
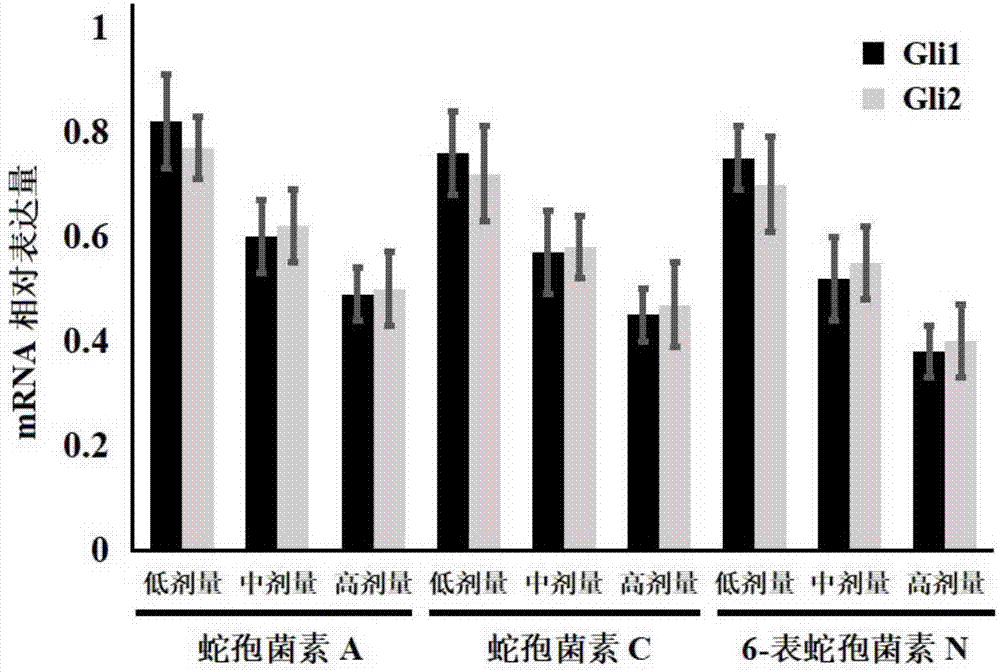
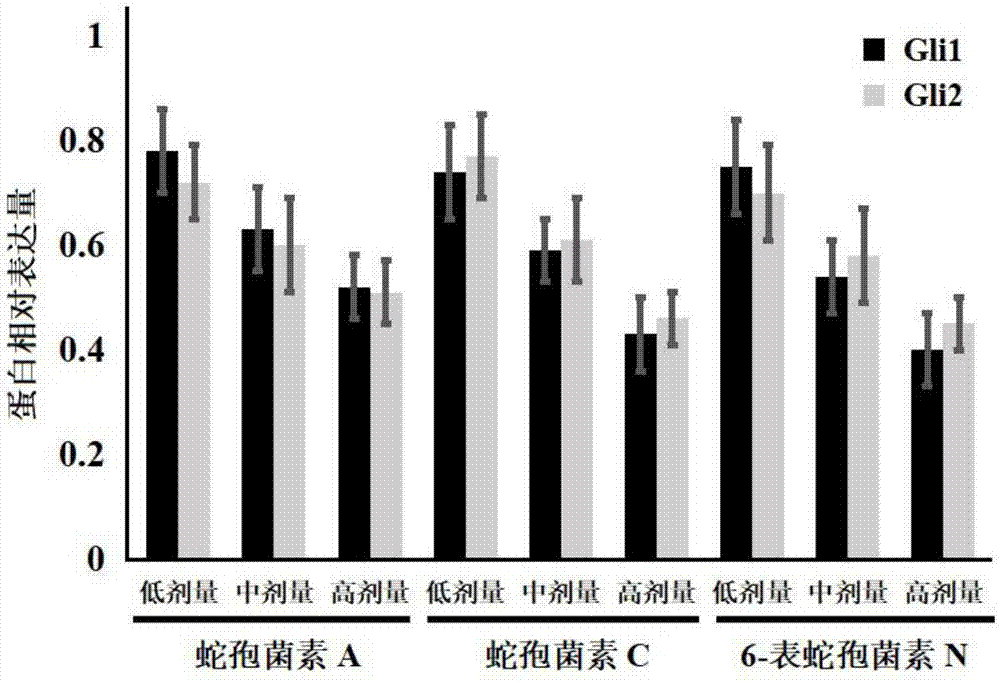
[0027] Example 1: Effects of Ophioporin A, C and 6-Epichoporin N on the Proliferation of Human Liver Cancer HepG2 Cells
[0028] 1. Experimental materials
[0029] Human liver cancer HepG2 cell line was purchased from Shanghai Jikai Gene Technology Co., Ltd.;
[0030] DMEM medium, fetal bovine serum and PBS were purchased from Gibco Company; TRIzol was purchased from Invitrogen Company; the reverse transcription kit was purchased from Fermentas Company of the United States, and the fluorescent quantitative PCR kit was purchased from TaKaRabao Bioengineering (Dalian) Co., Ltd.; cell lysate , SDS-PAGE protein loading buffer (5×), BCA protein concentration test kit, 20×TPBS buffer, etc. were purchased from Jiangsu Biyuntian Biotechnology Research Institute, PVDF membrane was purchased from Millipore, USA; CCK-8 kit Purchased from Tongren Institute of Chemistry in Japan; Gli1, Gli2 and GAPDH primers were synthesized by Shanghai Sangon Bioengineering Company;
[0031] Rabbit anti...
Embodiment 2
[0064] Example 2: Effects of Ophioporin A, C, and 6-Epichoporin N on the Proliferation of Human Liver Cancer SMMC-7721 Line Cells
[0065] 1. Experimental materials
[0066] Human liver cancer SMMC-7721 cell line was purchased from Shanghai Jikai Gene Technology Co., Ltd.;
[0067] DMEM medium, fetal bovine serum and PBS were purchased from Gibco Company; TRIzol was purchased from Invitrogen Company; the reverse transcription kit was purchased from Fermentas Company of the United States, and the fluorescent quantitative PCR kit was purchased from TaKaRabao Bioengineering (Dalian) Co., Ltd.; cell lysate , SDS-PAGE protein loading buffer (5×), BCA protein concentration test kit, 20×TPBS buffer, etc. were purchased from Jiangsu Biyuntian Biotechnology Research Institute, PVDF membrane was purchased from Millipore, USA; CCK-8 kit Purchased from Tongren Institute of Chemistry in Japan; Gli1, Gli2 and GAPDH primers were synthesized by Shanghai Sangon Bioengineering Company;
[006...
Embodiment 3
[0101] Example 3: Effects of Ophioporin A, C and 6-Epichoporin N on the Growth of Liver Cancer Transplanted Tumors in Nude Mice
[0102] HepG2 cells in a well-growth logarithmic growth phase were digested with trypsin, centrifuged and counted with trypan blue, and made 1×10 with normal saline 7 cells / mL of cell suspension were inoculated at 0.5 cm of the right axilla of nude mice, and each nude mouse was inoculated with 0.1 mL of cell suspension. Use a vernier caliper to measure the diameter of the transplanted tumor in nude mice, and wait until the tumor grows to about 100mm 3 At that time, the nude mice were randomly divided into cephalosporin A group, cephalosporin C group, 6-epichosporin N group and control group, with 10 mice in each group. At the same time, the nude mice in each group began to be administered, and the administration regimen of the cephalosporin A group, the cephalosporin C group, and the 6-epichosporin N group was: intragastric administration, 25 mg / kg / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com