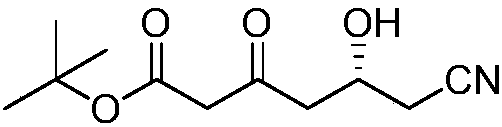
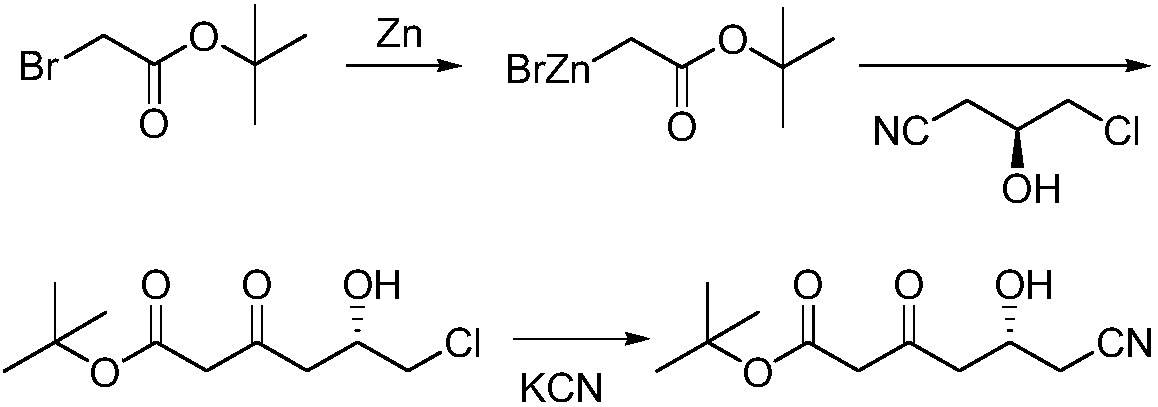
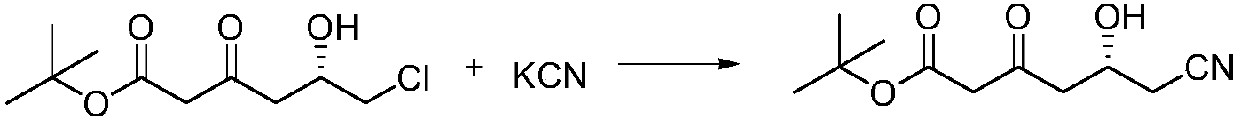Preparation method of (R)-6-cyan-5-hydroxy-3-tert-butyl carbonyl hexanoate
A technology for tert-butyl carbonyl hexanoate and tert-butyl acetate is applied in the field of preparation of -6-cyano-5-hydroxy-3-carbonyl hexanoate tert-butyl ester, and can solve the problem of handling difficulties, difficult recovery of liquid nitrogen, Environmental pollution and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under the protection of nitrogen, 116 grams of tert-butyl acetate and 160 grams of 1,8-diazabicycloundec-7-ene were added to 500 mL of 2-methyltetrahydrofuran, the temperature was raised to reflux, and the reaction was stirred for 5 hours. Slowly lower the temperature to 30°C to prepare solution A. Dissolve 150 g of (R)-4-cyano-3-hydroxybutyrate ethyl ester in 100 mL of 2-methyltetrahydrofuran to prepare solution B. Mix solution A and B Add the solution dropwise to 100mL 2-methyltetrahydrofuran through two constant flow pumps, stir vigorously, control the drop rate of solution A to be about 5 times that of solution B, control the reaction temperature at 20°C through the drop rate, and finish the drop at the same time. After the dropwise reaction was stirred for 1 hour, 35 mL of water was added dropwise, and the mixture was separated into layers. The organic phase was concentrated to obtain 220 g of tert-butyl R)-6-cyano-5-hydroxy-3-carbonylhexanoate, the optical purity ...
Embodiment 2
[0025] Under the protection of nitrogen, 116 grams of tert-butyl acetate and 152 grams of 1,8-diazabicycloundec-7-ene were added to 500 mL of 2-methyltetrahydrofuran, the temperature was raised to reflux, and the reaction was stirred for 1 hour. Slowly lower the temperature to 25°C to prepare solution A. Dissolve 157 g of (R)-4-cyano-3-hydroxybutyrate ethyl ester in 100 mL of 2-methyltetrahydrofuran to prepare solution B. Mix solution A and B Add the solution dropwise to 100mL 2-methyltetrahydrofuran through two constant flow pumps, stir vigorously, control the drop rate of solution A to be about 5 times that of solution B, control the reaction temperature at 30°C through the drop rate, and finish the drop at the same time. After the dropwise reaction was stirred for 5 hours, 70 mL of water was added dropwise, and the mixture was separated into layers. The organic phase was concentrated to obtain 214 grams of tert-butyl R)-6-cyano-5-hydroxy-3-carbonylhexanoate, and 680 ml of s...
Embodiment 3
[0027] Under nitrogen protection, 116 grams of tert-butyl acetate and 152 grams of reclaimed 1,8-diazabicycloundec-7-ene were added to 500 mL of reclaimed 2-methyltetrahydrofuran, heated to a reflux state, and stirred React for 1 hour, slowly lower the temperature to 25 ° C, prepare A solution, dissolve 157 g of (R)-4-cyano-3-hydroxybutyrate ethyl ester in 100 mL of recovered 2-methyltetrahydrofuran, and prepare B solution , Add solution A and solution B dropwise into 100mL of recovered 2-methyltetrahydrofuran through two constant flow pumps, stir vigorously, control the drop rate of solution A to be about 5 times that of solution B, and control the reaction temperature through the drop rate At 30°C, dropwise was completed at the same time, and after the dropwise reaction was stirred for 3 hours, 70 mL of water was added dropwise, and the layers were statically separated. The organic phase was concentrated to obtain 195 grams of tert-butyl R)-6-cyano-5-hydroxy-3-carbonylhexano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com