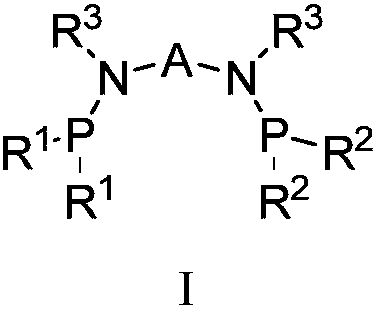
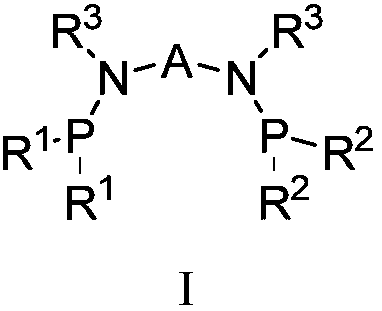
Catalyst system for ethylene selective oligomerization and ethylene oligomerization method
A catalyst and selective technology, applied in the field of catalysis, can solve the problem of low total selectivity of 1-hexene and 1-octene, and achieve the effects of low cost, simple synthesis and long catalyst life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] In one embodiment of the present invention, the preparation method of catalyst system comprises the following steps:
[0081] Components a, b, and c are pre-mixed or directly added to the reaction system for in-situ synthesis. That is to say, the preparation of the catalyst is to pre-mix the ligand a, the transition metal compound b, and the activator c connected by a bridging group containing a heteroatom; Body a, transition metal compound b, and activator c are directly added to the reaction system for in-situ synthesis;
[0082] The reaction mode of the ligand a, the transition metal compound b and the activator c connected by a bridging group containing a heteroatom described in the general formula I can be reacted in a liquid phase, such as reacting under the action of a solvent. Selected solvents such as toluene, benzene and their derivatives, etc.; can also be used for solid phase reactions; and can also be used to generate catalysts through in-situ reactions du...
Embodiment 1
[0091] 1. Preparation of ligand: N, N'-bis(diisopropylphosphino)-1,1-dimethyl-N, N'-diphenylsilanediamine (C 26 h 44 N 2 P 2 Si)
[0092] (1) Preparation of Lithium Anilide
[0093] in the N 2 Add dehydrated THF (200ml) and anilinolithium (9.31g, 0.1mol) into a fully replaced stirred 500ml reactor, stir well and cool to -78°C with liquid nitrogen. Use a 100ml syringe to extract n-butyllithium hexane solution (41.6ml, 2.4mol / L), slowly add it dropwise to the above solution while stirring, keep stirring at -78°C for 1h, then rise to room temperature and continue stirring for 1 hour, then vacuum pump The solvent was removed, n-hexane (100ml) was added, stirred and dispersed, and then filtered. The obtained filtrate was vacuum-extracted at room temperature to obtain 9.97g (0.098mol, 97.8%) of the product.
[0094] (2) prepare 1,1-diisopropyl-N-phenylphosphine amine (C 12 h 20 NP)
[0095] in N 2 In an atmosphere glove box, dissolve lithium anilide (4.95g, 0.050mol) in de...
Embodiment 2
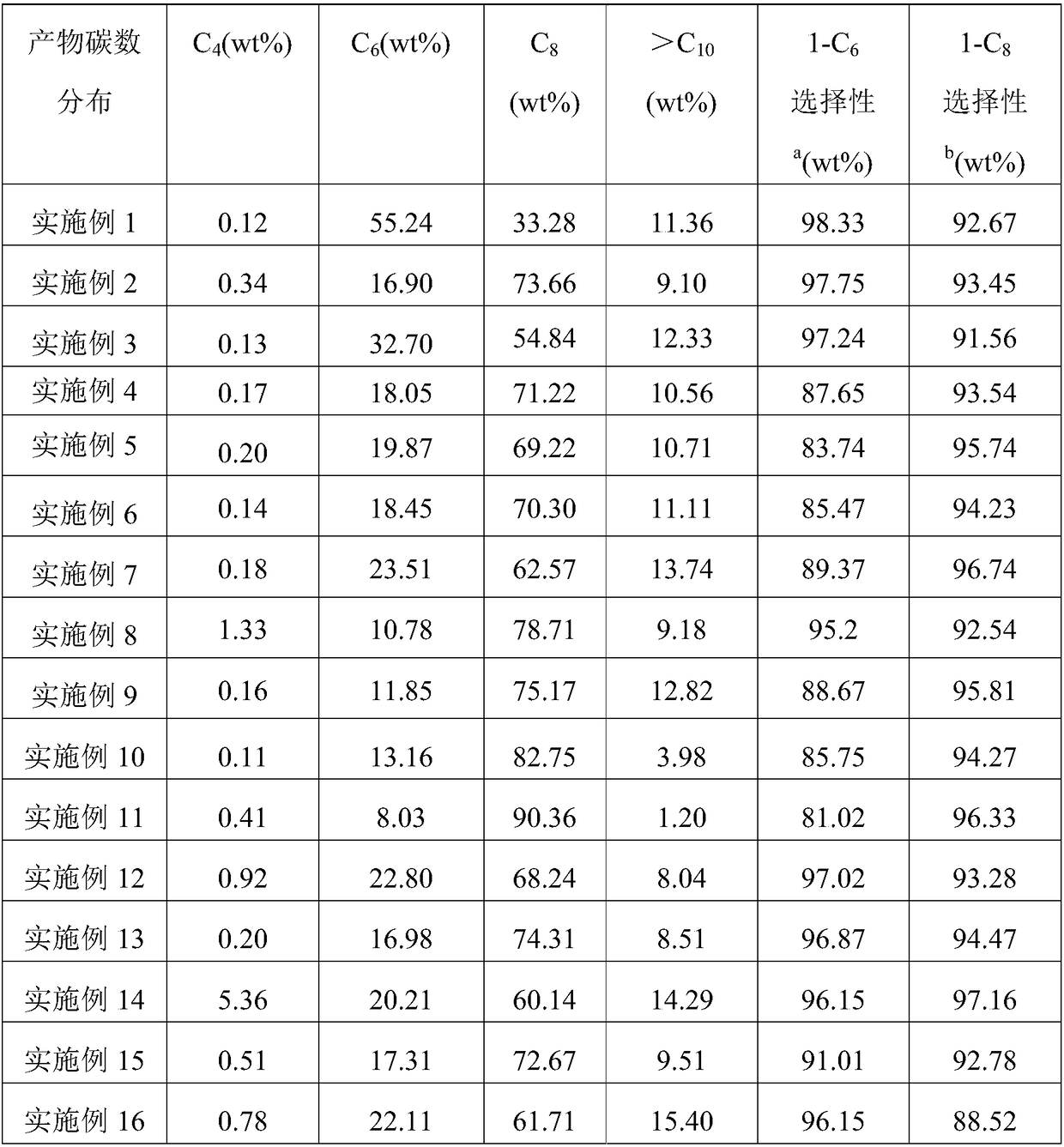
[0104] With embodiment 1. The difference is that R 1 , R 2 Both are phenyl, R 3 For cyclopentyl. Obtain 83.2g of oligomerization product, catalyst activity is 5.04×10 6 g oligomer / mol Cr·h. The distribution of the oligomerization products is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com