Cross-linkable amphiphilic natural polysaccharide and application thereof
A natural polysaccharide and amphiphilic technology, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, aerosol delivery, etc., can solve the problems of poor stability and achieve efficient delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
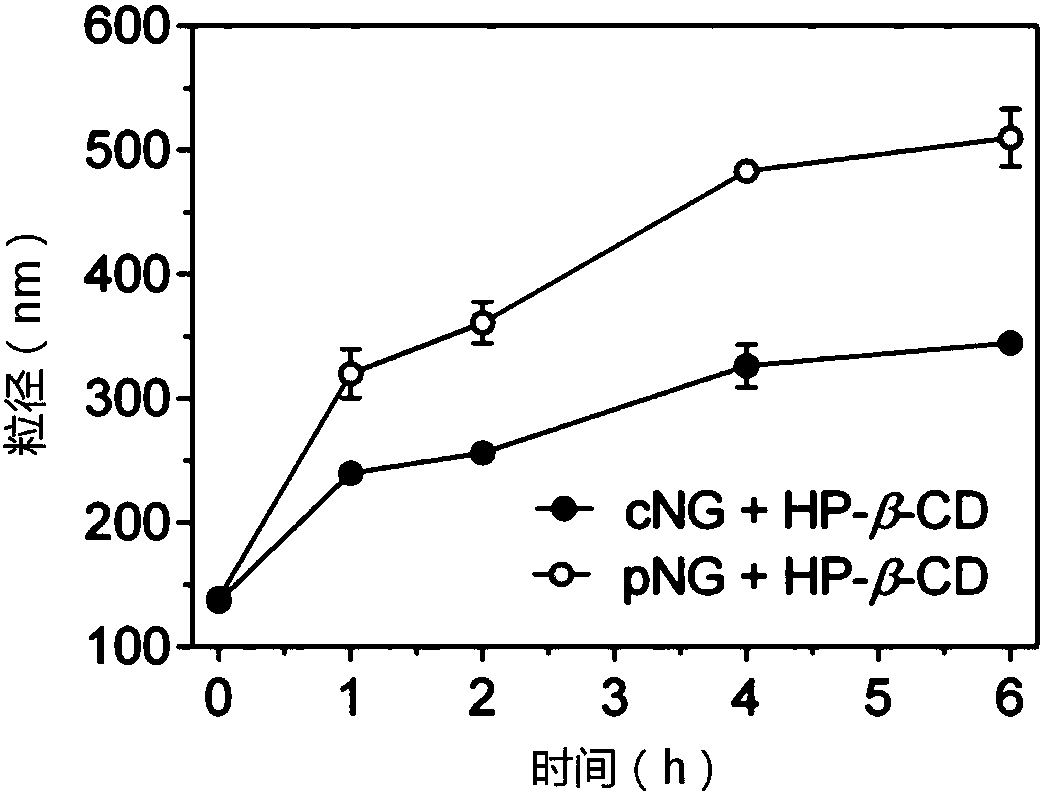
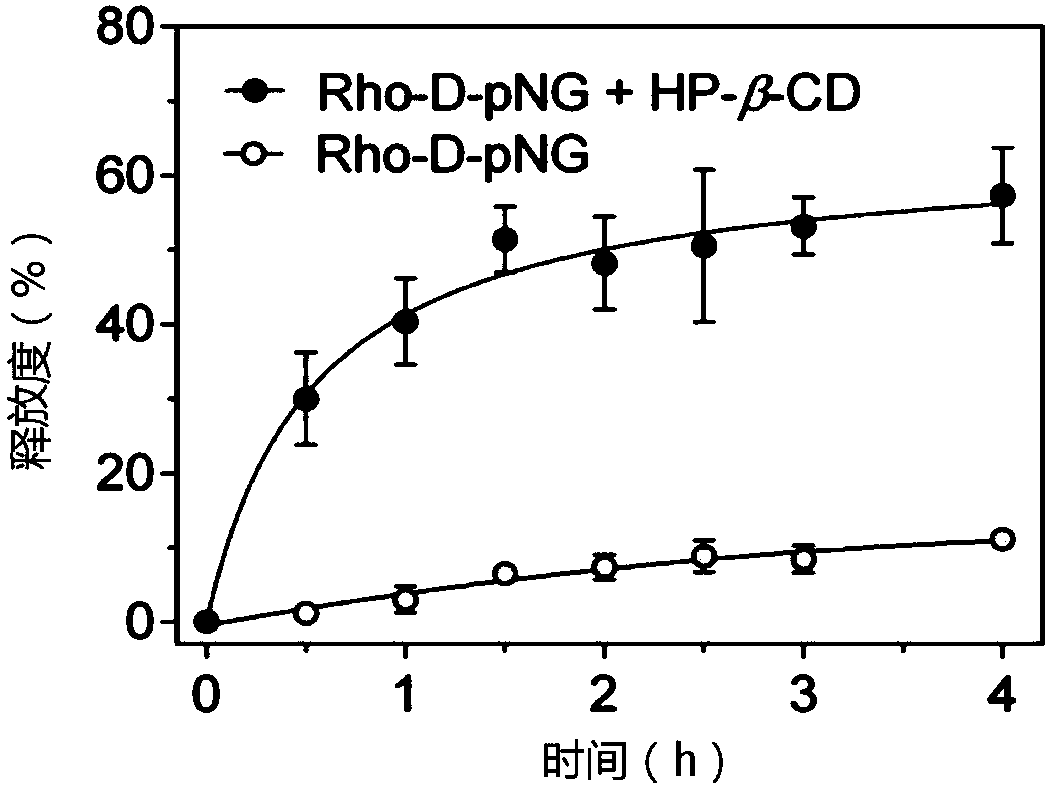
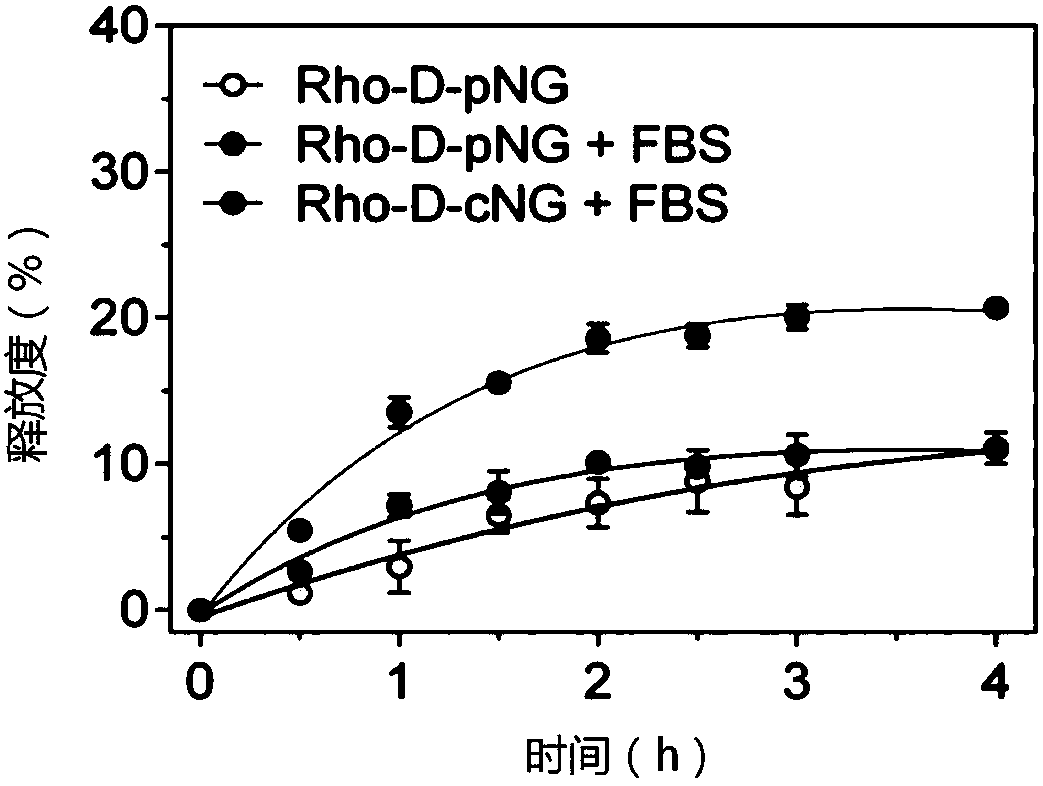
[0040] The blank nanogel was prepared by probe ultrasonic method. The cross-linkable amphiphilic hyaluronic acid was dissolved in the aqueous solution, and the probe was ultrasonicated for 10 minutes in an ice bath with an ultrasonic intensity of 240W. The loading of protein is carried in the presence of a small amount of organic solvent, including methanol, ethanol, dimethyl sulfoxide, etc., preferably dimethyl sulfoxide. In the present invention, multiple proteins are used to investigate the efficiency of protein loading, and the mass ratio of nanogel and protein is 1:0.2 to 1:1. Taking 1:0.2 as an example, the encapsulation rate of various model proteins is as high as 85-99%. .
[0041] It has been reported in the literature that the modification of hyaluronic acid with cholesterol alone can be used for protein loading (T.Nakai, T.Hirakura, Y.Sakurai, T.Shimoboji, M.Ishigai, K.Akiyoshi. Injectable hydrogel for sustained protein release by salt- induced association of hyal...
Embodiment 1
[0043] 1. Synthesis of hyaluronic acid double-modified with methacrylic acid and cholesteryl hexamethylenediamine
[0044] The first step, the preparation of cholesteryl hexamethylenediamine:
[0045] Cholesterol chloroformate dissolved in dichloromethane was added dropwise to 1,6-hexanediamine dissolved in dichloromethane, the molar ratio of 1,6-hexanediamine to cholesterol chloroformate was 10:1. After the dropwise addition, react at room temperature for 2 h. After the reacted product was washed with water, the organic phase was collected and dried with anhydrous sodium sulfate for half an hour, filtered with suction to remove anhydrous sodium sulfate, and a light yellow solid was obtained as a crude product after rotary evaporation. The purified product was purified by chromatographic column to obtain cholesterol hexamethylenediamine (product 1).
[0046]
[0047] The second step, the synthesis of methacrylic acid modified hyaluronic acid:
[0048] Add 1.6mL 2-methacr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com