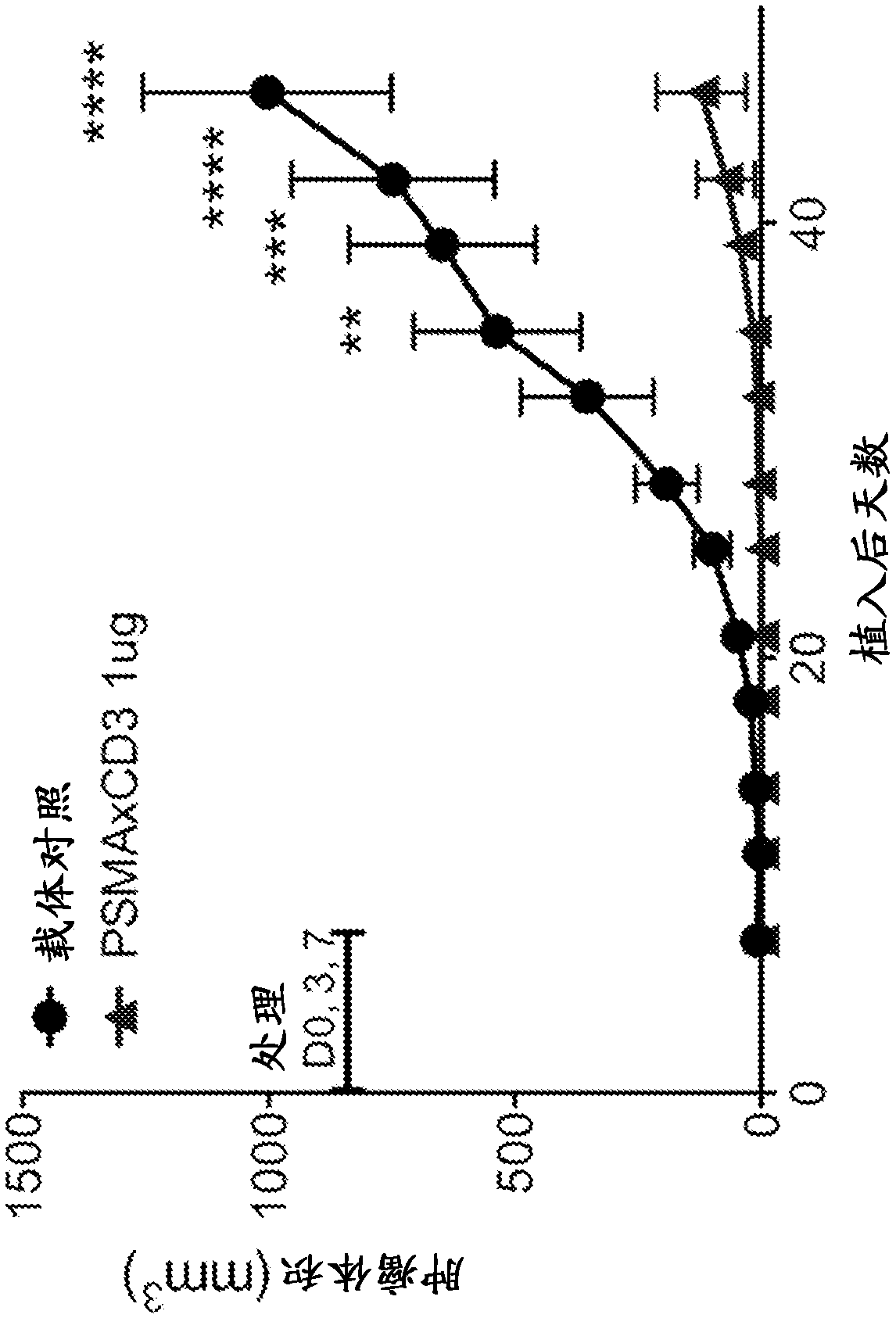
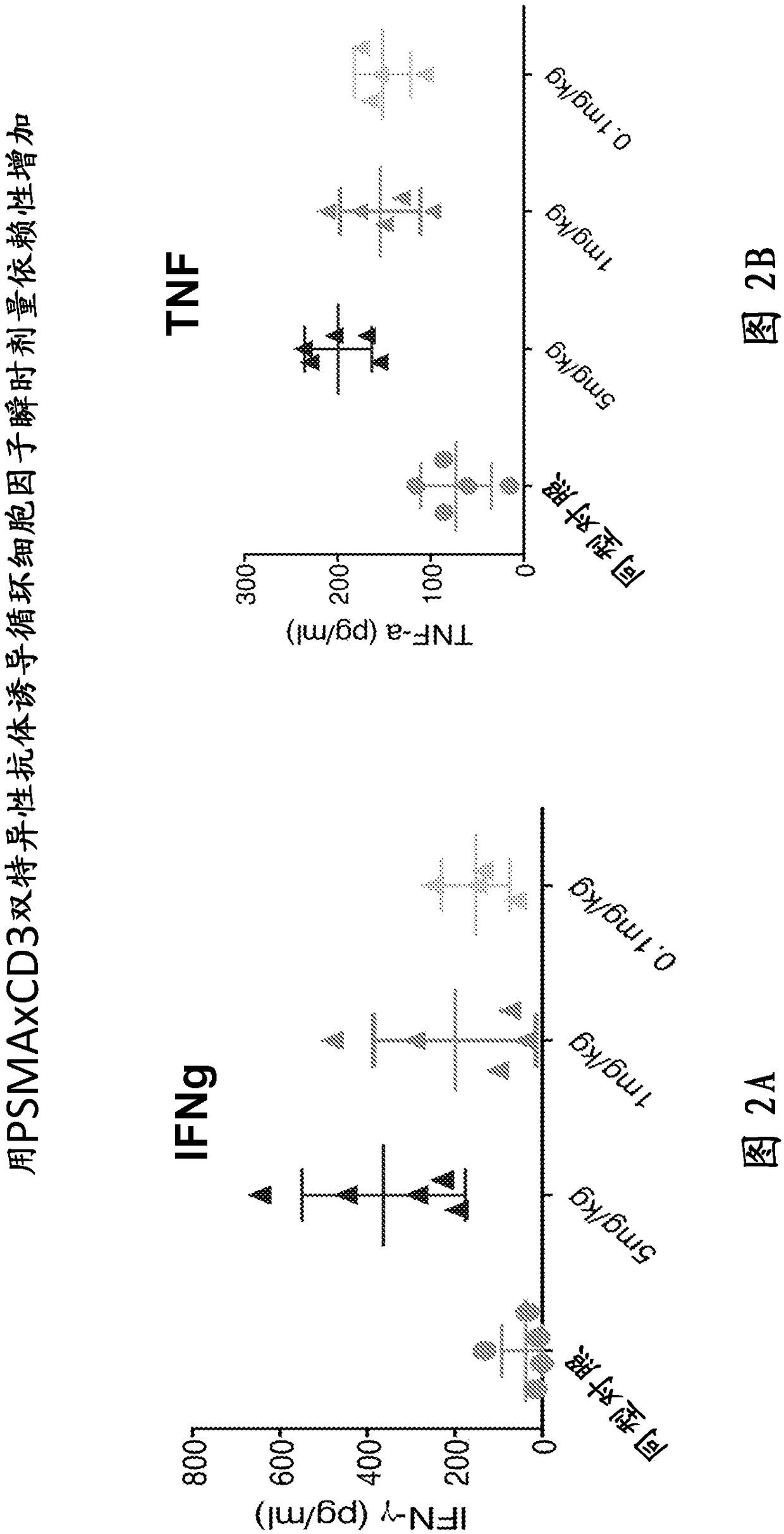
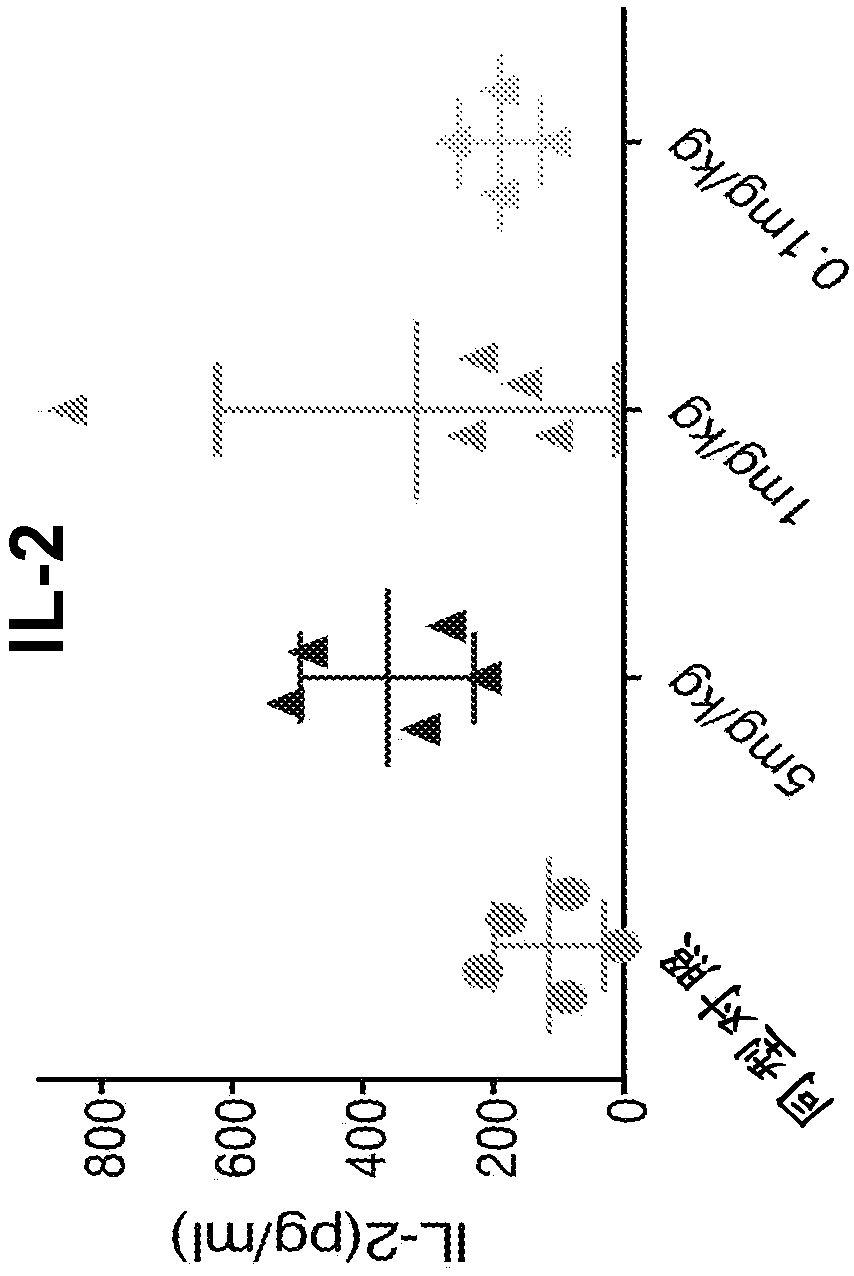
Anti-psma antibodies, bispecific antigen-binding molecules that bind psma and cd3, and uses thereof
An antigen-binding molecule and bispecific technology, applied in anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody, anti-animal/human immunoglobulin, etc., can solve toxicity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0246] Example 1: Production of Anti-PSMA Antibodies
[0247] Resistivity is obtained by immunizing genetically modified mice with human PSMA antigen, or by immunizing engineered mice containing DNA encoding human immunoglobulin heavy chain and kappa light chain variable regions with human PSMA antigen. PSMA antibody.
[0248] Human prostate cancer cells (LNCaP, Manassas, Virginia, USA) immunized mice. After immunization, splenocytes were harvested from each mouse and either (1) fused with mouse myeloma cells to maintain their viability and form hybridomas and screened for PSMA specificity, or (2) with N-terminal 6- His-tagged human PSMA (R&D, catalog number 4234-ZN) was used as a sorting reagent for binding and identifying reactive antibodies for B cell sorting (as described in US2007 / 0280945A1) (antigen-positive B cells).
[0249] Chimeric antibodies to PSMA with human variable regions and mouse constant regions were initially isolated. Antibodies are characterized and ...
Embodiment 2
[0251] Example 2: Amino Acid and Nucleic Acid Sequences of Heavy Chain and Light Chain Variable Regions of Anti-PSMA Antibody
[0252] figure 1 Amino acid sequence identifiers for selected heavy and light chain variable regions and CDRs of anti-PSMA antibodies of the invention are set forth. The corresponding nucleic acid sequence identifiers are set forth in Table 2.
[0253] Table 1: Amino Acid Sequence Identifiers
[0254]
[0255] Table 2: Nucleic acid sequence identifiers
[0256]
Embodiment 3
[0257] Example 3: Binding affinity and kinetic constant of human monoclonal anti-PSMA antibody obtained by surface plasmon resonance
[0258] In this example, the ability of anti-PSMA antibodies to bind to human PSMA was assessed. Binding affinity and kinetic constants of anti-PSMA antibodies to soluble human PSMA protein were determined by surface plasmon resonance at 37°C using an antibody capture format. The results are shown in Tables 3 and 4. Assays were performed on a Biacore T-200 instrument (GE Healthcare).
[0259] Briefly, capture purification by amine coupling with a monoclonal mouse anti-human Fc antibody (GE, #BR-1008-39) or a monoclonal goat anti-mouse Fc antibody (GE, #BR-1008-38) Anti-PSMA antibody, derivatized CM5Biacore sensor surface. In 0.01M HEPES, 0.15M NaCl, 2mM Ca 2+ , 2mM Mg 2+ , 0.05% v / v surfactant P20pH7.4 in HBSP++ buffer for binding studies. Different concentrations of expressed human PSMA with an N-terminal hexahistidine tag (6h.hPSMA, R&D) p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com