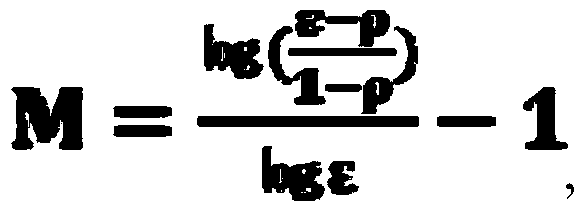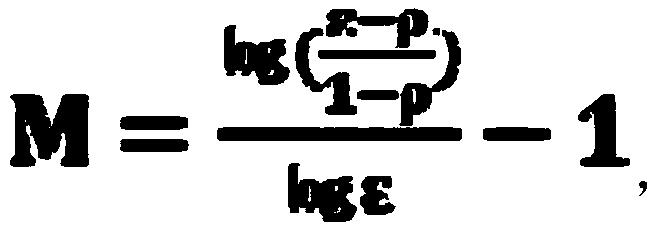Method for extracting high-purity lithium salt from lithium-containing brine
A technology for extracting lithium and brine, applied in the field of liquid-liquid extraction, can solve the problems of low product purity, inability to continue normal production, unstable process, etc., and achieve good practicability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The lithium-containing brine to be treated is deboronated brine, wherein the initial lithium concentration is 0.3 mol / L, and the magnesium concentration is 5.5 mol / L.
[0062] Use 3M LiCl solution as the exchange liquid to wash the exchange section (also known as the enrichment section), set to reach a lithium salt purity of 99.9% (i.e. Xp=99.9%), full reflux, do not take the product until the equilibrium is taken when starting The final concentration of the lithium salt is obtained as the product. According to the reflux extraction formula, when the separation coefficient of lithium and magnesium is calculated as 50, the theoretical number of stages N=1.766. The exchange section we actually operate is 2 stages. The analysis results show that the lithium salt The purity is 99.95%.
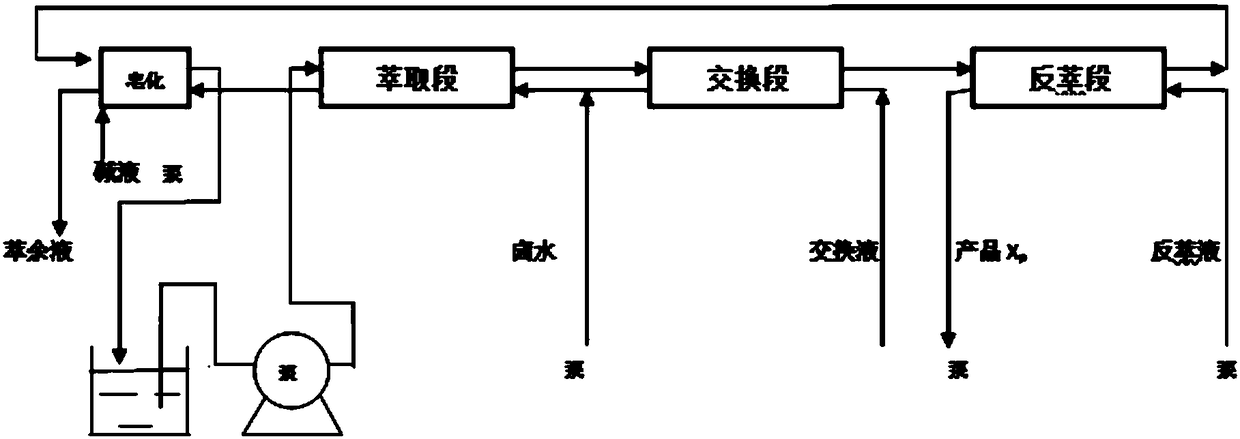
[0063] The process flow diagram of the present embodiment extracting high-purity lithium salt can be found in figure 1 .
[0064] (1) adding the hydrochloric acid solution of ferric chlori...
Embodiment 2
[0071] The lithium-containing brine to be treated is deboronated brine, wherein the initial lithium concentration is 0.3 mol / L, and the magnesium concentration is 5.5 mol / L.
[0072] Use 3M LiCl solution as the exchange liquid to reflux, set the purity of lithium salt to 99.9% (i.e. Xp=99.9%), and the reflux flow to 0.25LεX0. When the separation coefficient of lithium and magnesium is calculated as 50, the theoretical number of stages N=2.2609 , Our actual operating exchange section is grade 4, and the analysis results show that the purity of lithium is 99.99%.
[0073] The technical process of extracting high-purity lithium salt in this embodiment is the same as that in Embodiment 1.
Embodiment 3
[0075] The lithium-containing brine to be treated is deboronated brine, wherein the initial lithium concentration is 0.3 mol / L, and the magnesium concentration is 5.5 mol / L.
[0076] Use 4M LiCl solution as the exchange liquid to reflux, set the purity of lithium salt to 99.9% (that is, Xp=99.9%), and the reflux flow to 0.25LεX0. When the separation coefficient of lithium and magnesium is calculated as 50, the theoretical number of stages N=2.2609 , Our actual operating exchange section is grade 3, and the analysis results show that the purity of lithium is 99.95%.
[0077] The technical process of extracting high-purity lithium salt in this embodiment is the same as that in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com