Second-order culture process technique for producing suspended MDCK cell relevant vaccine
A technology for vaccine production and suspension cells, applied in animal cells, vertebrate cells, urinary tract/kidney cells, etc., to achieve the effect of simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
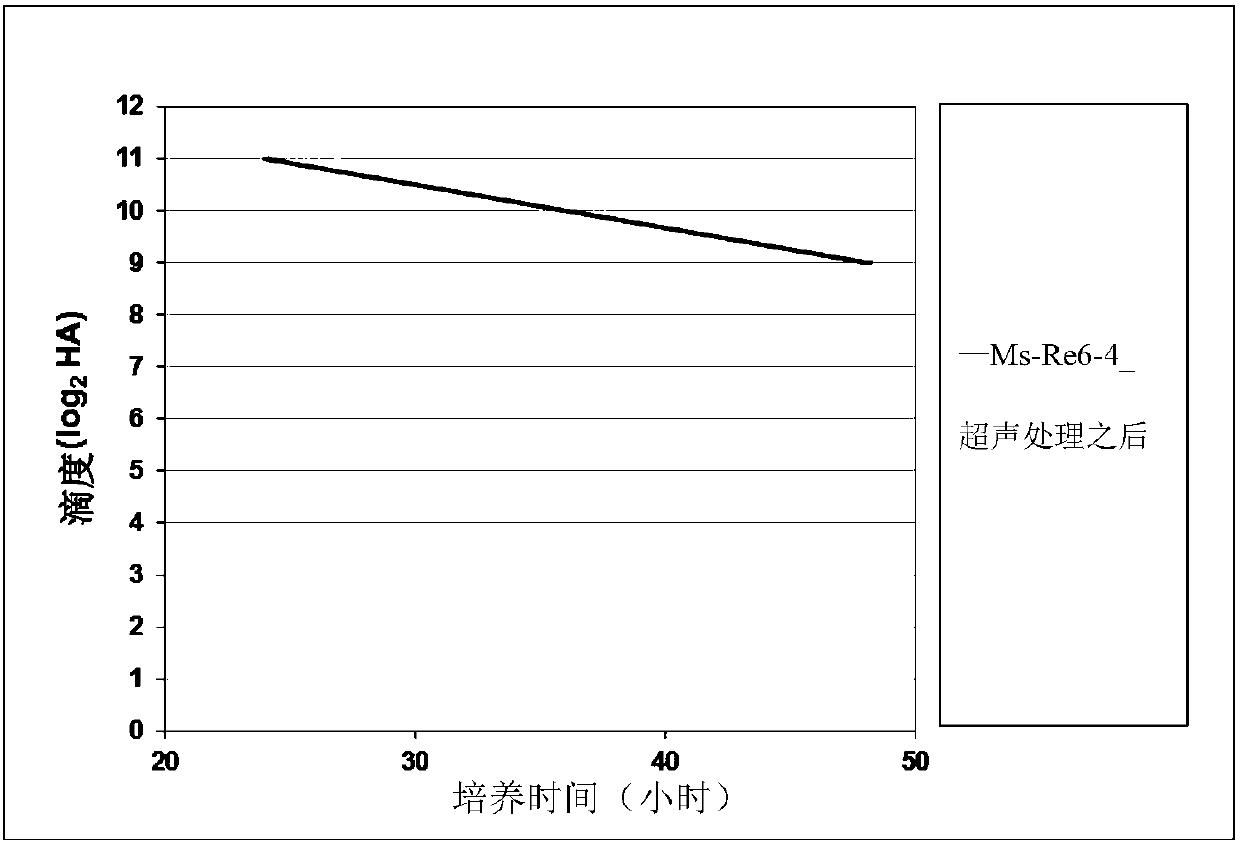
[0051] Embodiment 1 avian influenza virus is tested in the secondary culture process test of MDCK suspension cell
[0052] 1. Experimental materials
[0053] Cell line: MDCK cell line derived from ATCC, independently domesticated by Jianshun Biotech, can grow in suspension. The acclimatization condition is to reduce the serum of MDCK adherent cells to less than 5% and pass on for more than 3 generations, and then carry out suspension acclimatization culture, and the acclimatization condition is 5% CO 2 , the shaker speed was 120RPM / min, and the MDCK cell lines capable of suspension growth were screened out.
[0054] Medium: CD MDCK SFM serum-free medium (JSB, DP197, Cat. No. 88022-197).
[0055] Avian influenza virus Re-6 (Valneva Company, France).
[0056] 2. Method
[0057] Include the following steps
[0058] (1) Recovery and passage of cells
[0059] (1) Preparation of culture medium: prepare liquid culture medium CD MDCK SFM 20L according to the instructions, and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com