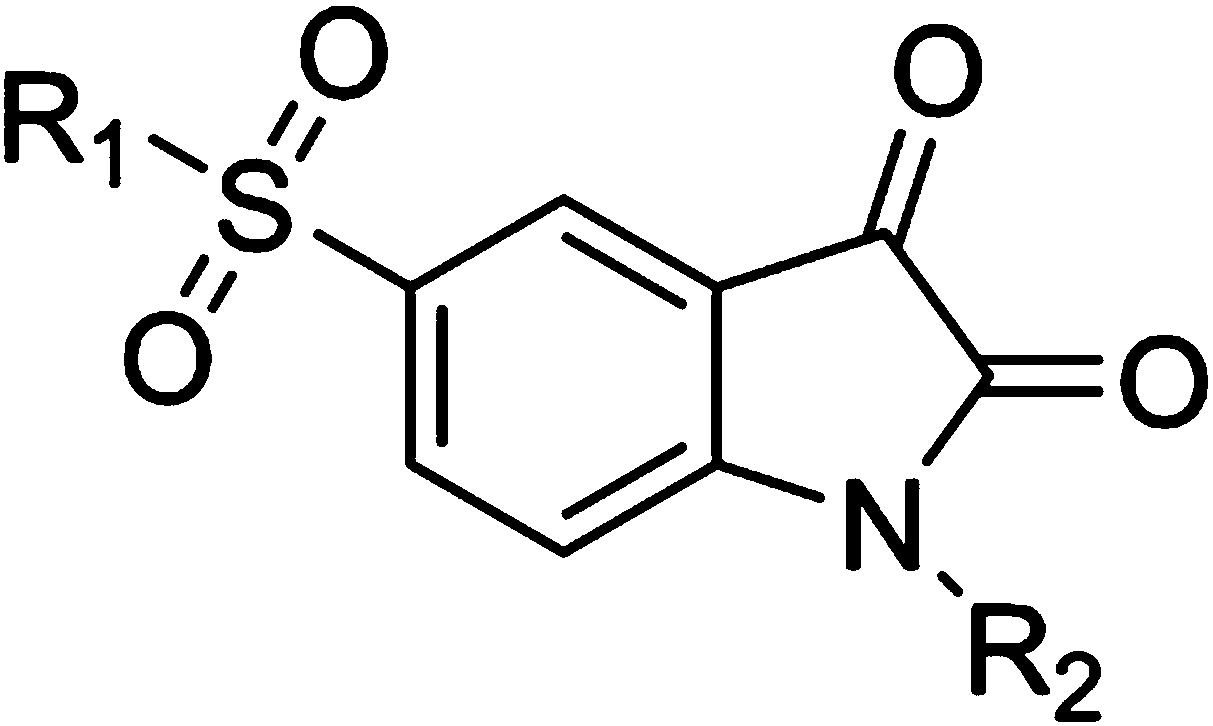
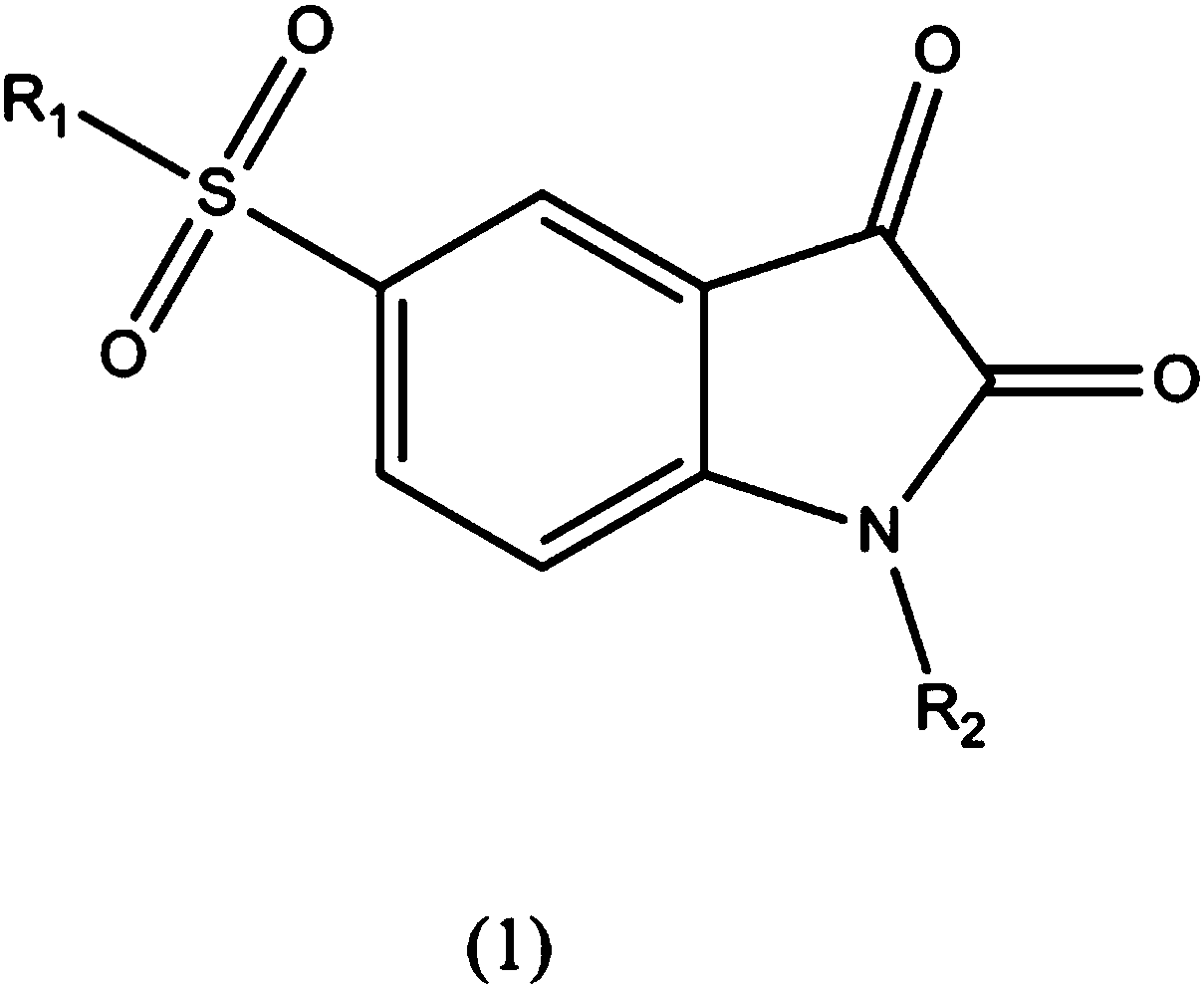
Isatin-5-sulfonamide inhibitor with inhibition effect on MLL (mixed lineage leukemia) key protein
A technology of sulfonamides and isatins, applied in the field of isatin-5-sulfonamide inhibitors, can solve the problems of lack of specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
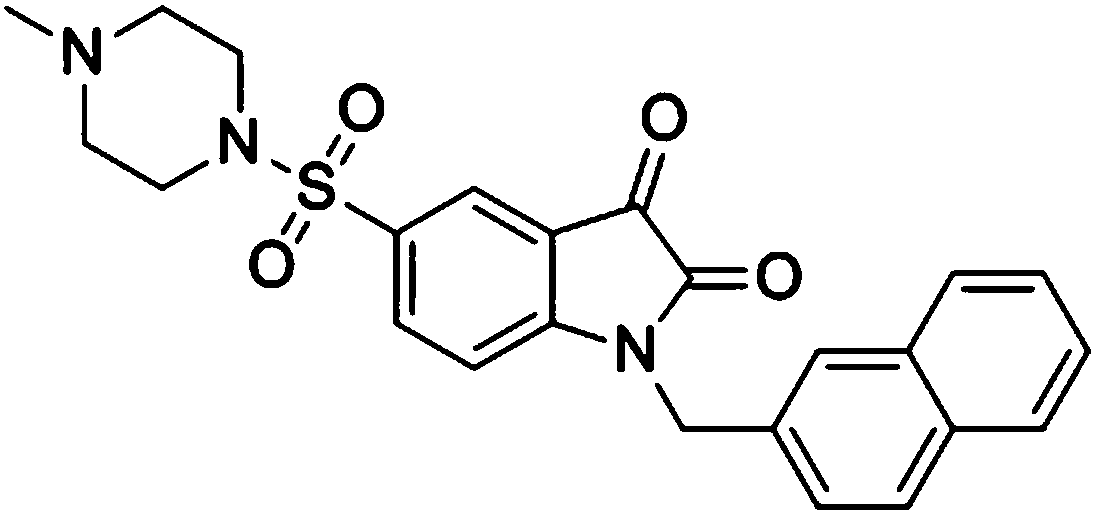
[0040] 1-(2-Naphthylmethyl)-5-(N-methylpiperazinylsulfonyl)isatin
[0041]
[0042] Specific steps:
[0043]1. Under nitrogen and ice bath, 20.0ml (302mmol) chlorosulfonic acid was added dropwise to 3.6g (24.5mmol) isatin. Then, the mixture was heated to 70°C for 3 hours. The mixture was cooled to room temperature and poured carefully into 100 g of ice. The resulting yellow solid was filtered and washed with cold water. After dissolving in ethyl acetate and drying with Na2SO4, the mixture was purified by column chromatography (petroleum ether and ethyl acetate) to give compounds 1 (3.8 g) and 2 (410 mg). 1 or 2 can react with secondary amines, and 2 can directly generate compound 4 in high yield. The obtained precipitate was used for the next reaction without separation. Compound 1 was a light yellow powder, and Compound 2 was a yellow powder.
[0044] 2. Under nitrogen, the mixture of 1 and 2 (2.0 mmol) was dissolved in 6 ml of dry THF at 0°C. Then, in 1 ml of THF so...
Embodiment 2
[0073] The equilibrium dissociation constants of isatin-5-sulfonamide compounds were measured according to the equilibrium dissociation constant test method between the above-mentioned compounds and DOT1L, and the compounds with inhibitory activity on DOT1L were obtained as follows.
[0074]
[0075] KD value is 6.75e-6 (6.78μM)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com