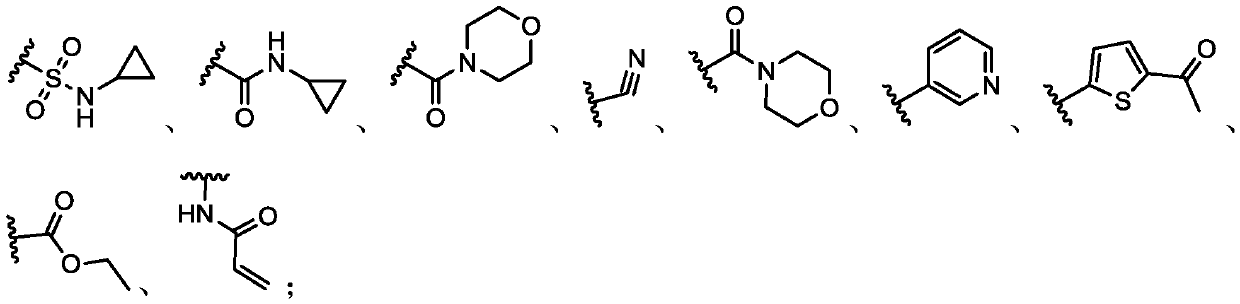
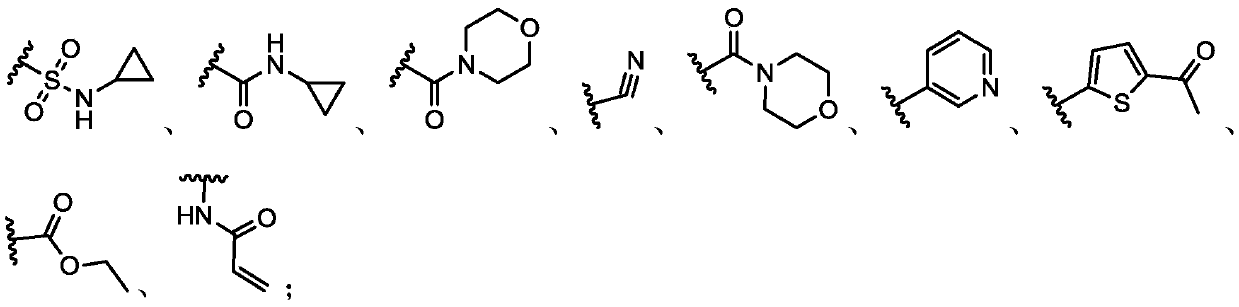
Histone lysine methyltransferase NSD2 inhibiting small-molecule compound and application thereof
A small molecule compound, lysine methyl technology, applied in the direction of drug combination, organic chemistry, antineoplastic drugs, etc., can solve the problems of no high activity NSD2 small molecule inhibitors, limited research, etc., to achieve good medicinal potential, The preparation method is simple and the energy consumption is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Compound 1: Compound N-cyclopropyl-3-(3-(1-methyl-1H-benzo[d][1,2,3]triazole-5)ureido)benzenesulfonamide
[0034] The synthetic route is as follows:
[0035]
[0036] Concrete synthetic steps are as follows:
[0037] At 0°C, N-methyl-4-nitrobenzene-1,2-diamine (A01a) (1.13g, 6.76mmol) was dispersed in 22ml of water, and then 5.6ml of concentrated hydrochloric acid was added to form a strongly acidic mixture An aqueous solution of sodium nitrite (568 mg, 8.23 mmol) dissolved in 10 ml was added dropwise. Keep stirring at 0°C for 4 hours, then slowly rise to room temperature, filter and wash the filter cake with cold water to obtain the off-white compound 5-nitrobenzo(1-methyl)triazole (A01b).
[0038] Then A01b (950mg, 5.3mmol) was dispersed in about 50ml EtOH:H 2In the solvent system of O=2:1, ammonium chloride (570mg, 10.6mmol) was added and the temperature was raised. When the temperature rose to 60°C, iron powder (1190mg, 21.2mmol) was added, and the temperatu...
Embodiment 2
[0043] Compound 2: N-cyclopropyl-3-(3-(1-methyl-1H-benzo[d][1,2,3]triazole-5)ureido)benzamide
[0044] The synthetic route is as follows:
[0045]
[0046] Other specific synthetic steps were the same as the above-mentioned examples, and compound 2 was prepared with a yield of 61.2%.
[0047] The MS (ESI) data of compound 2 are as follows:
[0048] MS(ESI)351.3[M+H] + ; 1 H NMR (400MHz, DMSO-d 6 )δ8.91(s,1H),8.89(s,1H),8.41(d,J=3.6Hz,1H),8.20(s,1H),7.86(s,1H),7.77(d,J=8.8 Hz,1H),7.64(d,J=8.0Hz,1H),7.51(d,J=7.6Hz,1H),7.40(d,J=7.6Hz,1H),7.35(t,J=7.6Hz, 1H),4.28(s,3H),2.91–2.79(m,1H),0.73–0.66(m,2H),0.58-0.57(m,2H).
Embodiment 3
[0050] Compound 3: 1-(1-methyl-1H-benzo[d][1,2,3]triazole-5)-3-(3-(morpholine-4-carbonyl)phenyl)urea
[0051] The synthetic route is as follows:
[0052]
[0053] Other specific synthetic steps were the same as those in the above examples, and compound 3 was prepared with a yield of 68.3%.
[0054] The MS (ESI) data of compound 3 are as follows:
[0055] MS(ESI)381.1[M+H] + ; 1 H NMR (400MHz, DMSO-d 6 )δ8.96(s,1H),8.90(s,1H),8.19(d,J=1.2Hz,1H),7.77(d,J=8.8Hz,1H),7.60(s,1H),7.54– 7.43(m,2H),7.36(t,J=7.6Hz,1H),7.01(d,J=7.6Hz,1H),4.27(s,3H),3.61(s,8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com