Monooxygenase mutant and preparation method and application thereof
A monooxygenase and monooxygenase conversion rate technology, applied in the field of genetic engineering, can solve the problems of large amount of enzyme added, low enzyme stability, poor soluble expression, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Monooxygenase mutant of single point mutation (M25A)
[0055] Construction of monooxygenase mutant genes:
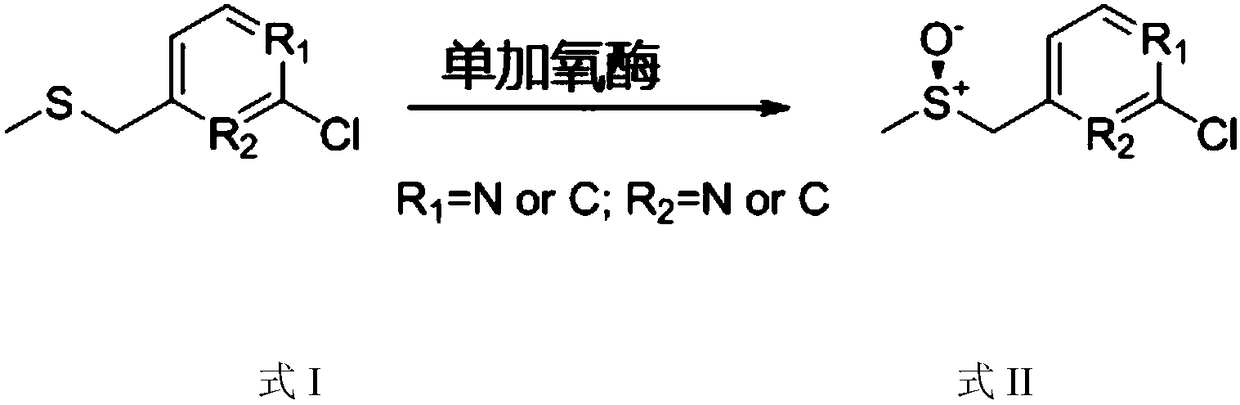
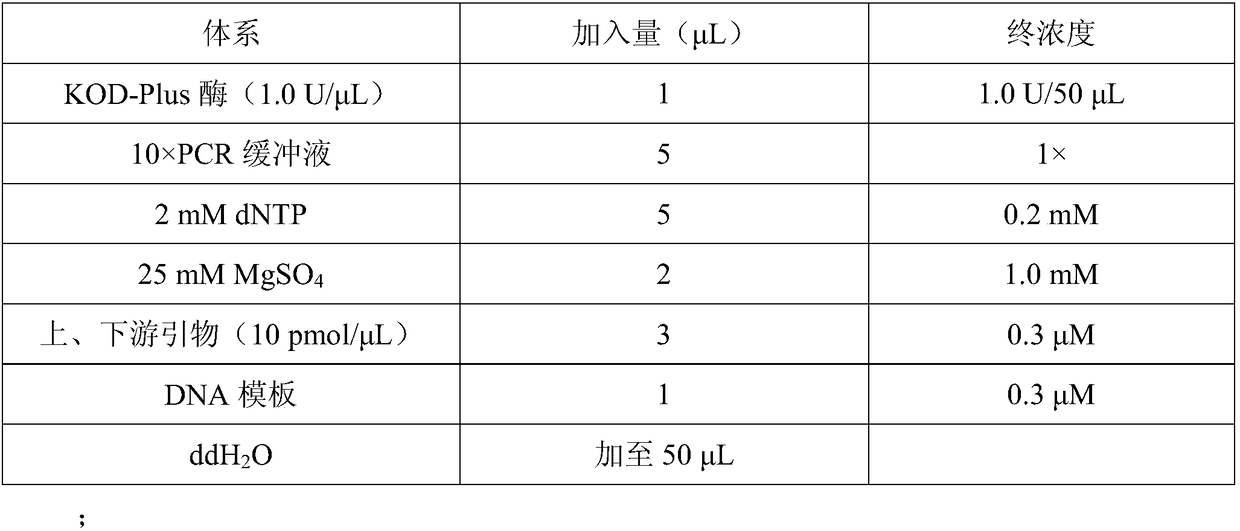
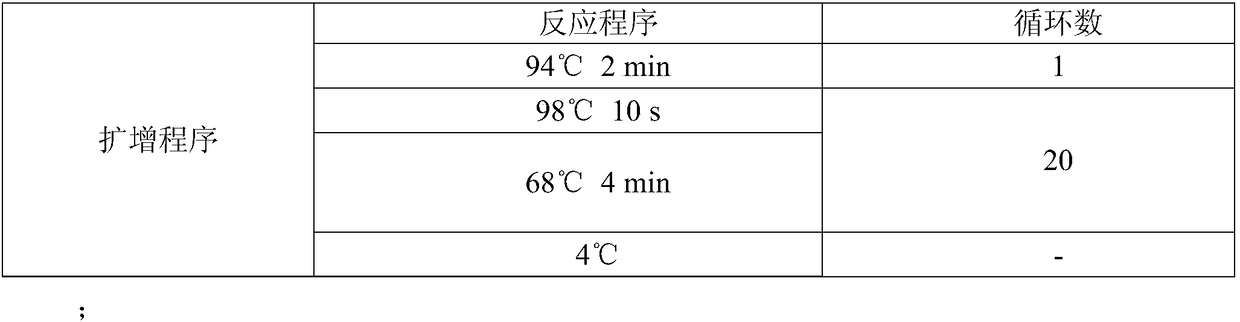
[0056] In order to improve the activity, stability and soluble expression of monooxygenase CHMO (its amino acid sequence is SEQ ID NO.1, and its encoding nucleotide sequence is SEQ ID NO.2) derived from Brachymonas petroleovorans, select properties, reduce the amount of enzyme used, and mutate the M25A site respectively. The specific steps are as follows:
[0057] The nucleotide sequence shown in the SEQ ID NO.2 is as follows:
[0058] ATGAGTAGCAGCCCGAGCAGCGCCATCCACTTTGACGCCATTGTGGTGGGTGCCGGTTTTGGCGGCATGTATATGCTGCACAAGCTGCGCGACCAGCTGGGCCTGAAAGTTAAAGTGTTCGACACCGCCGGTGGTATTGGTGGTACCTGGTACTGGAACCGCTATCCGGGTGCCCTGAGCGACACCCATAGCCACGTGTACCAGTACAGCTTCGATGAGGCCATGCTGCAGGAGTGGACATGGAAAAATAAATATCTGACCCAGCCGGAAATCCTGGCATATCTGGAATACGTGGCCGATCGTCTGGATTTACGCCCTGACATTCAGCTGAACACCACCGTTACCAGCATGCATTTTAACGAGGTGCACAATATCTGGGAAGTTCGCACCGATCGTGGCGGCTACTATACAGCACGCTTCATTGT...
Embodiment 2
[0069] Example 2 Monooxygenase Mutants of Single Point Mutation (P106R)
[0070] Perform site-directed mutation on P106R, the specific steps are as follows:
[0071] Introducing mutations: designing forward and reverse primers containing the P106R site, the forward and reverse primers are as follows:
[0072] Upstream primer (SEQ ID NO.5): cgtctggatttacgccgtgacattcagctgaac;
[0073] Downstream primer (SEQ ID NO.6): gttcagctgaatgtcacggcgtaaatccagacg;
[0074] Other methods and steps are the same as in Example 1.
Embodiment 3
[0075] Example 3 Monooxygenase Mutants of Single Point Mutation (R159L)
[0076] Introducing mutations: designing forward and reverse primers containing the R159L site, the forward and reverse primers are as follows:
[0077] Upstream primer (SEQ ID NO.7): cgaacatcccgggccttgagtcttttcaagg;
[0078] Downstream primer (SEQ ID NO.8): ccttgaaaagactcaaggcccgggatgttcg;
[0079] Other methods and steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com