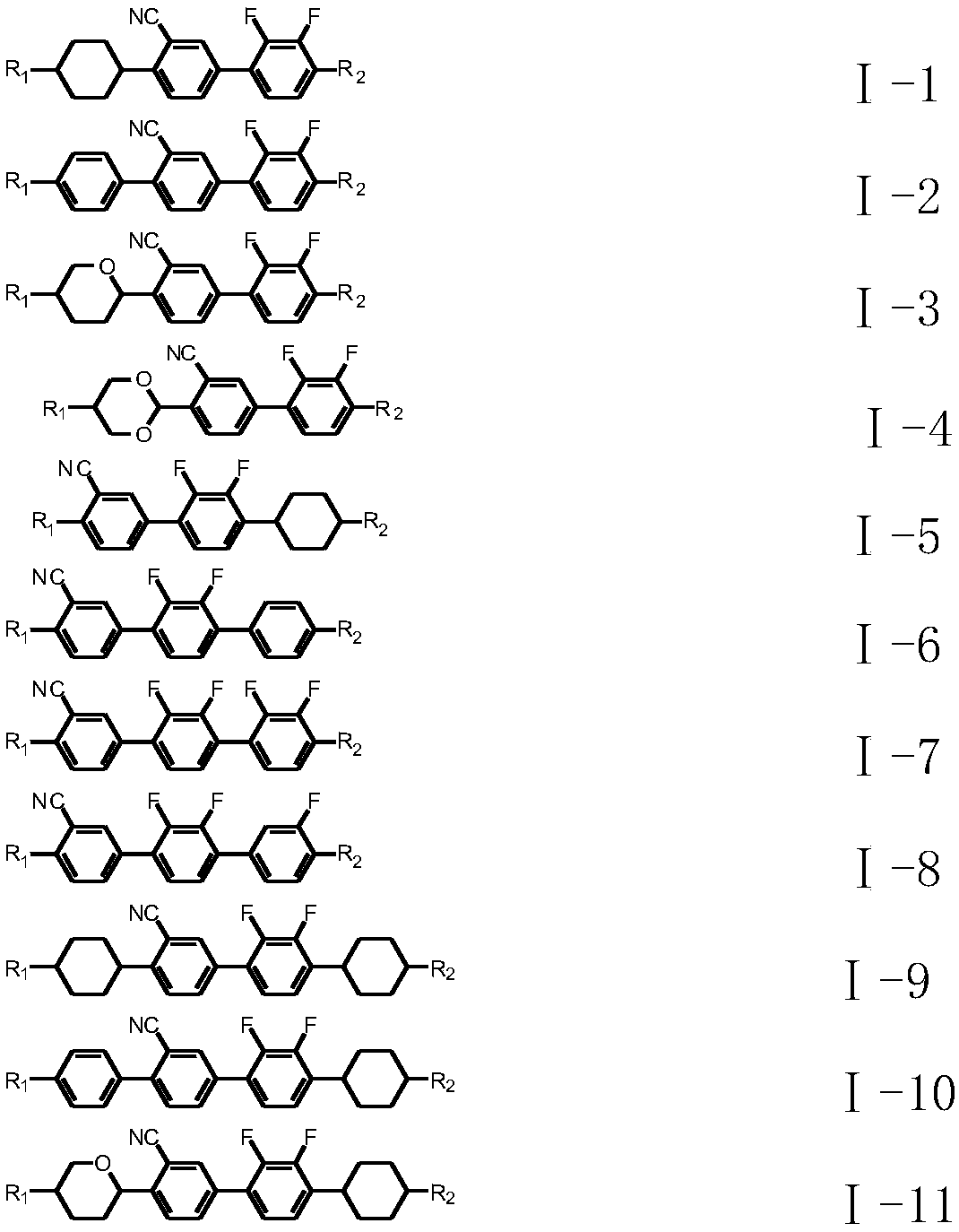
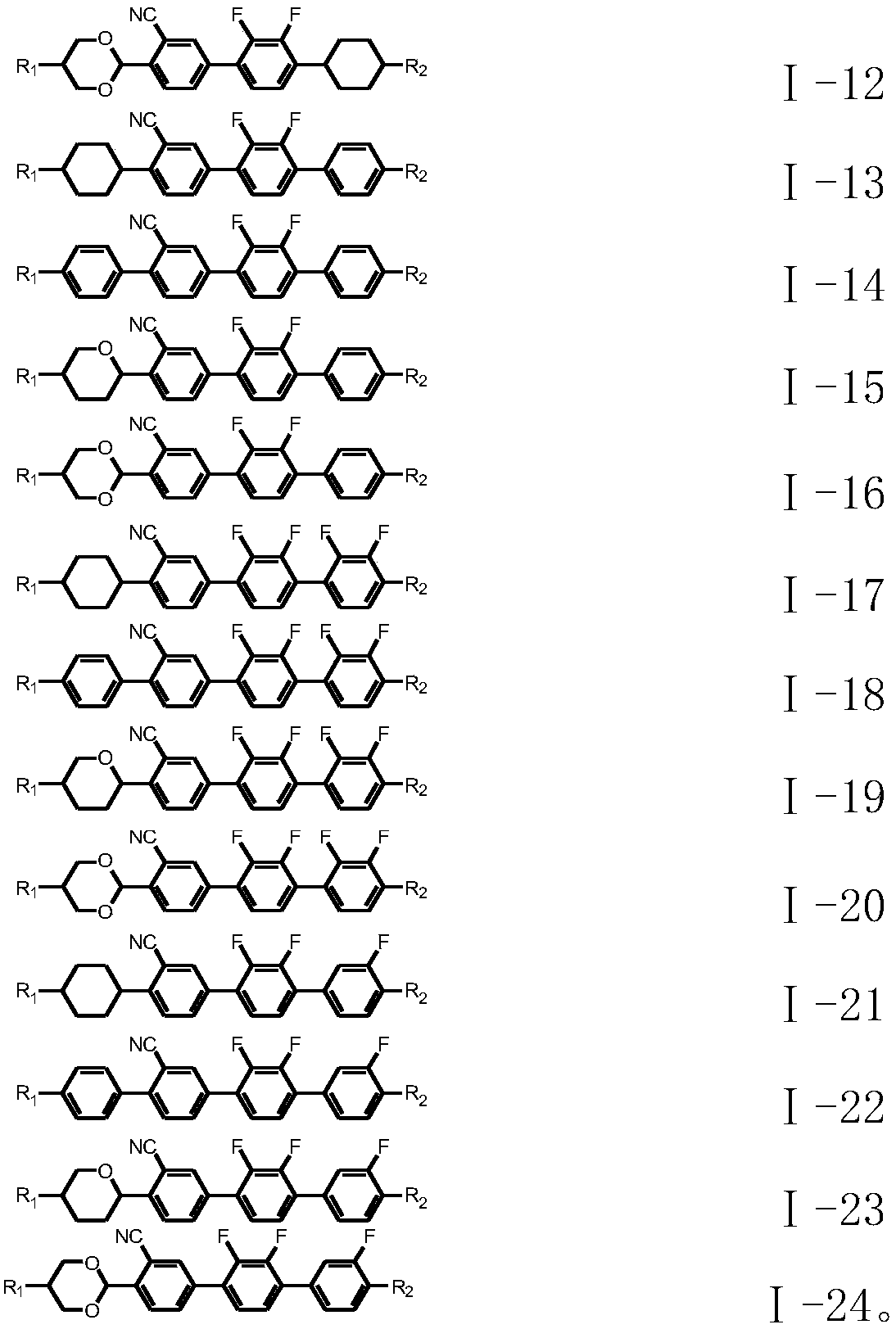Liquid crystal compound with negative dielectric anisotropy as well as preparation method and application thereof
A liquid crystal compound, anisotropic technology, applied in chemical instruments and methods, liquid crystal materials, etc., can solve problems such as long response time, achieve high-definition bright spots, good stability and UV resistance, large dielectric anisotropy absolute value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] a compound The preparation method, wherein the preparation route is as follows:
[0033]
[0034] The specific synthesis method is:
[0035] 1. 57.9g of 2,3-difluorobromobenzene was made into Grignard reagent with THF, cooled to -20°C, 19.1g of acetaldehyde was added dropwise to the Grignard reagent, the temperature was controlled at -20°C, and naturally rose to room temperature , stirred for 2 hours, spin-dried, added ammonium chloride aqueous solution, and extracted with EA; the EA phase was washed twice with saturated brine, and dried; added 300ml THF, 30ml TFA, stirred for 12h, and spin-dried to obtain 40.6g of the above compound 1.
[0036] 2. 40.6g of compound 1, 0.3g of palladium carbon in 300ml of ethanol, hydrogenated with hydrogen at normal pressure for 5h, filtered, and spin-dried to obtain 41.1g of the above-mentioned compound 2.
[0037] 3. Dissolve 41.1g of compound 2 in 200ml THF, cool to -78°C, add 119ml of butyllithium dropwise at -70°C, raise the...
Embodiment 2
[0045] a compound The preparation method, wherein the preparation route is as follows:
[0046]
[0047] The specific synthesis method is:
[0048] 1. Compound 3 was synthesized, and the synthetic method was the same as that of compound 3 in Example 1.
[0049] 2. 41g compound 3, 38.2g 1,2-difluorobromobenzene, 60g potassium phosphate, 0.5g tetrakistriphenylphosphorous palladium, 300ml toluene, stirred at 90°C for 8h under nitrogen protection; cooled, concentrated, added EA, and used Washed with saturated brine three times, dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain 48.3 g of compound 9.
[0050] 3. Dissolve 48.3g of compound 9 in 200ml of THF, cool to -78°C, add 84ml of butyllithium dropwise at -70°C, raise the temperature to -40°C, and stir for 1 hour. After cooling to -78°C, 48g of tributyl borate was added dropwise, and naturally rose to room temperature, and stirred for 6h. Add 200ml of water containing 40ml of concentrated hydrochlori...
Embodiment 4
[0070] a compound The preparation method, wherein the preparation route is as follows:
[0071]
[0072] The specific synthesis method is:
[0073] 1. Compound 13 was synthesized, and the synthetic method was the same as that of compound 13 in Example 2.
[0074] 2. 57g compound 13, 17g p-propylphenylboronic acid, 40g potassium phosphate, 0.5g tetrakistriphenylphosphorous palladium, 200ml toluene, stirred at 90°C for 8h under the protection of nitrogen; cooled, concentrated, added EA, washed with saturated brine 3 times, dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain 54.7 g of compound 22.
[0075] 3. 54.7g of compound 22, 360ml of ethanol, 0.1g of TBAF were stirred for 12 hours; spin-dried to obtain 43g of compound 23.
[0076] 4. 43g of compound 23, 8.7g of manganese dioxide, and 150ml of THF were stirred at room temperature for 12h. Filtered and spin-dried to obtain 42 g of compound 24.
[0077] 5. 42g of compound 24, 7.1g of hydroxylamine ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Clear point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com