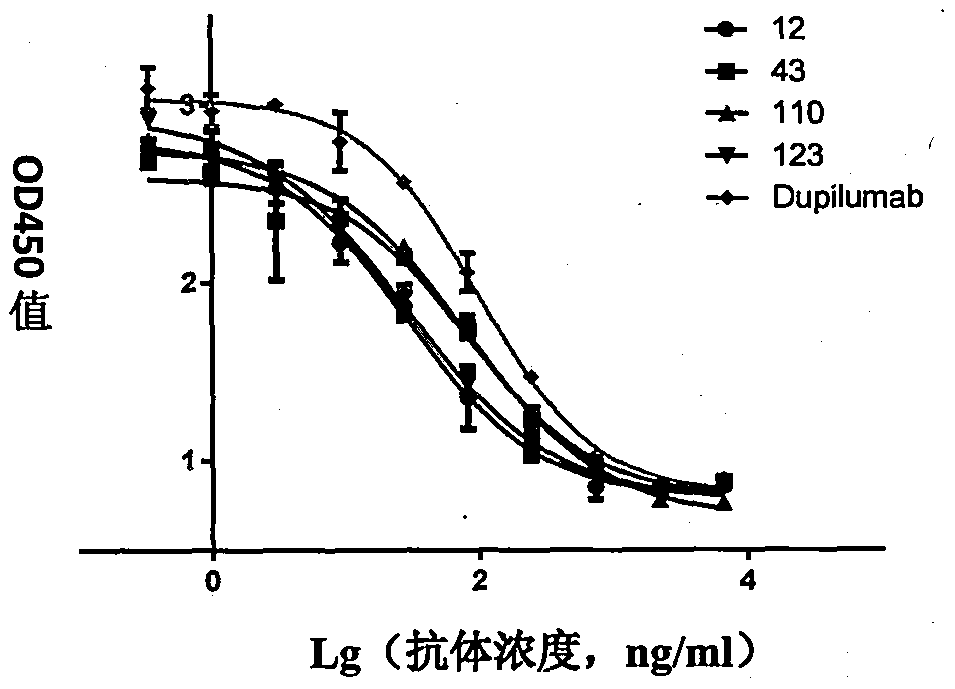
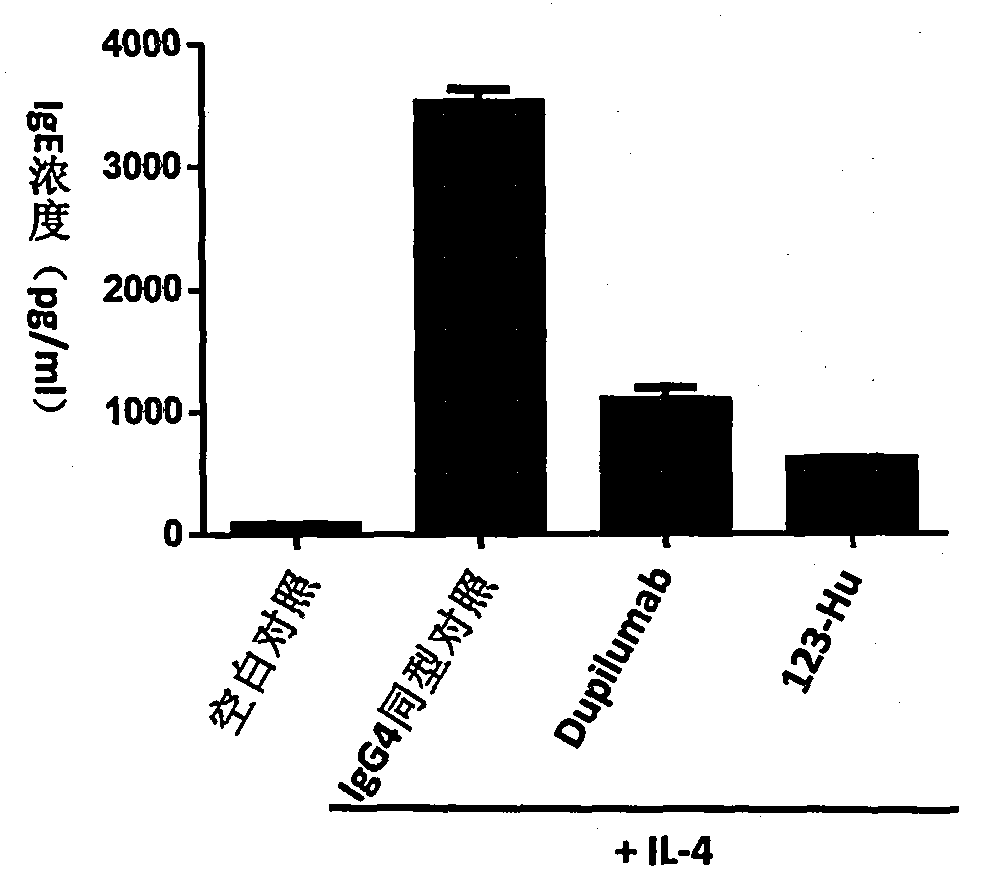
Anti-human interleukin-4 receptor alpha monoclonal antibody, preparation method and applications thereof
A monoclonal antibody, humanized technology, applied in the field of its preparation, anti-human interleukin-4 receptor α monoclonal antibody, can solve the problem that patients have no cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040]The preparation method of the anti-human IL-4Rα monoclonal antibody disclosed in the present invention comprises: cultivating the above-mentioned host cells under expression conditions, thereby expressing the anti-human IL-4Rα monoclonal antibody; isolating and purifying the anti-human IL-4Rα 4Rα monoclonal antibody. Using the above method, the recombinant protein can be purified into a substantially uniform substance, such as a single band on SDS-PAGE electrophoresis.
[0041] The method of affinity chromatography can be used to separate and purify the recombinant protein disclosed in the present invention. According to the characteristics of the used affinity column, conventional methods such as high-salt buffer, changing pH, etc. can be used to elute the protein bound to the affinity column. The fusion protein polypeptide on.
[0042] The humanized anti-human IL-4Rα monoclonal antibody disclosed in the present invention is obtained by the following method: using the ...
Embodiment 1
[0051] Example 1 Preparation of soluble IL-4Rα extracellular segment Fc tag and Flag tag antigen, positive antibody Dupilumab, IL-4, IL-13
[0052] The human IL-4Rα ectoantigen sequence was obtained from UniProt (UniProtKB-P24394). Sangon Biotech Co., Ltd. carried out codon optimization according to the codon usage preference of Cricetulus griseus and synthesized the N-terminal 26-232 amino acid fragment. Cloned into the pUC57 vector (from Sangon Biotechnology Co., Ltd.) to obtain pUC57-hIL4Rα-ECD. The constant region sequence of human IgG1 was synthesized according to the sequence of Secukinumab (see WHO Drug Information Vol. 23, No. 4, 2009. P342). The sequence was subcloned into pUC57 vector (from Sangon Biotechnology Co., Ltd.) to obtain pUC57-IgG1-CH. hIL4Rα-ECD and hFc (human Fc fragment) were spliced by PCR method to obtain hIL4Rα-ECD-hFc fragment. Using pUC57-hIL4Rα-ECD as a template, the Flag tag (DYKDDDDK) was inserted into the C-terminus of the hIL4Rα-ECD fragme...
Embodiment 2
[0059] Example 2 Immunization of hIL-4RαECD-Fc
[0060] 100 μg / mouse hIL-4RαECD-Fc antigen was diluted to 75 μl with normal saline, mixed with an equal volume of Freund’s complete adjuvant, and after complete phacoemulsification, the 4-5 week-old Balb / c mice (purchased Subcutaneous multi-point injection was performed from Shanghai Lingchang Biotechnology Co., Ltd., animal production license number: SCXK (Shanghai) 2013-0018). Three weeks later, 50 μg / mouse protein was also diluted to 75 μl and mixed with an equal volume of Freund’s incomplete adjuvant. After phacoemulsification was complete, the mice were subcutaneously immunized at multiple points, and the immunization was repeated two weeks later. One week after the third immunization, the tails of all mice were cut to collect blood to separate serum, and the serum titer was detected by ELISA coated with hIL-4Rα-Fc antigen. For mice with serum antibody titer > 10,000, pulse immunization was performed one week after blood co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com