Combined Biomarker Measurements of Fibrosis
A technology for fibrotic diseases and binding capacity, which can be used in biological testing, measuring devices, biomaterial analysis, etc., and can solve problems such as undetermined binding epitopes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
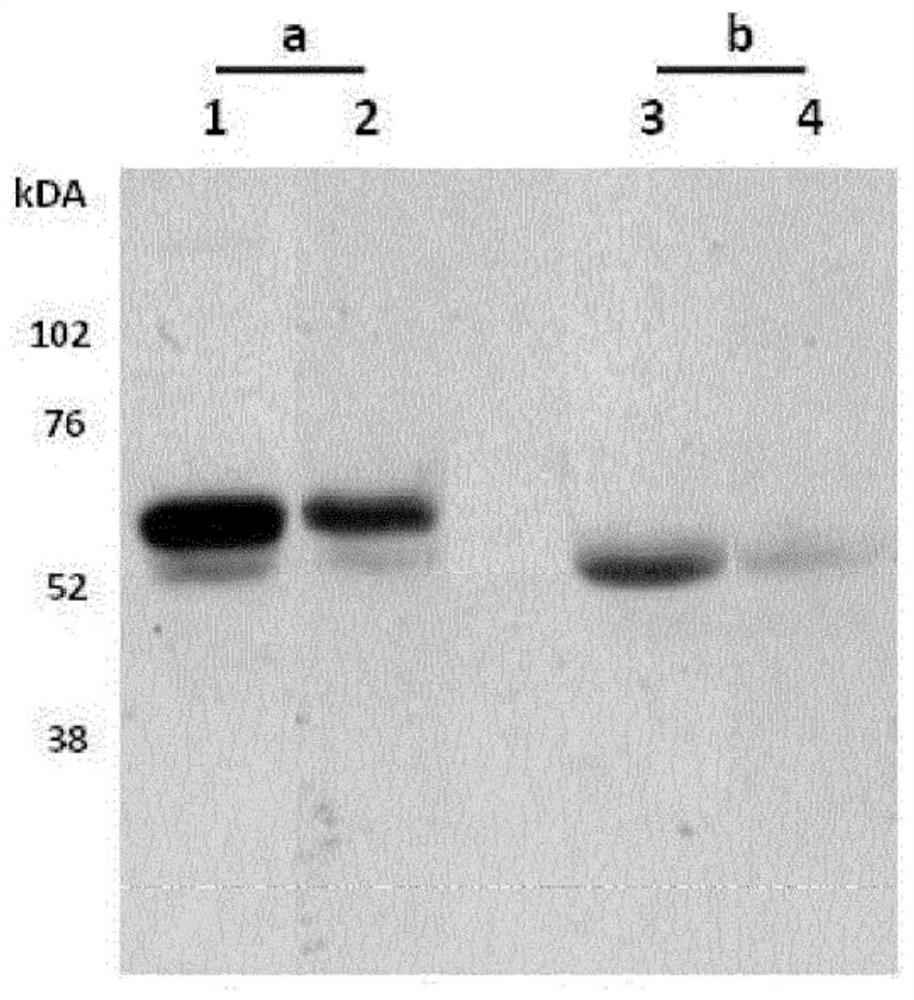
[0066] Example 1 - Monoclonal Antibody NB61-N62
[0067] Production of monoclonal antibodies
[0068] The N-terminal propeptide sequences of type III collagen were aligned between human, rat, and mouse species, and homology between species and uniqueness among other ECM proteins were selected by protein blasting. The amino acid sequence 145'-CPTGPQNYSP-'153 (SEQ ID NO: 6) in the PIIINPα1 chain is 100% homologous between human and rat ( figure 1 ). Monoclonal initiation was initiated by subcutaneously immunizing 4-5 week-old Balb / C mice with 200 μL of emulsified antigen and 50 μg of the PIIINP neoepitope C-terminal sequence (OVA-CGG-CPTGPQNYSP (SEQ ID NO: 10)) using Freund's incomplete adjuvant. Antibody production. Immunizations were repeated every 2 weeks until a stable serum titer level was reached. The mouse with the highest serum titer was selected for fusion. Mice were rested for one month and then boosted intravenously with 50 μg of the PIIINP neoepitope C-terminal...
Embodiment 2
[0076] Example 2 - PRO-C3 ELISA using NB61N-62
[0077] Supernatants from antibody-producing hybridomas were collected and monoclonal antibodies were purified using HiTrap affinity columns (GE Healthcare LifeScience, Little Chalfont, Buckinghamshire, UK) and Lightning-Link according to the manufacturer's instructions. TM The HRP Conjugation Kit (Innova Biosciences, Babraham, Cambridge, UK) was labeled with HRP.
[0078] The process of PRO-C3 competitive ELISA was as follows: the biotinylated peptide biotin-CGG-CPTGPQNYSP (SEQ ID NO: 11) dissolved in coating buffer (50 mM PBS-BTE+10% sorbitol, pH7.4) 96-well streptavidin-coated ELISA plates from Roche, cat. 11940279 were coated, incubated at 20° C. in the dark for 30 minutes, and then washed in wash buffer (20 mM Tris, 50 mM NaCl, pH 7.2). Thereafter, 20 μL of peptide calibrators or samples were added to appropriate wells, followed by 100 μL of HRP-conjugated monoclonal antibody dissolved in incubation buffer (50 mM PBS-BTB +...
Embodiment 3
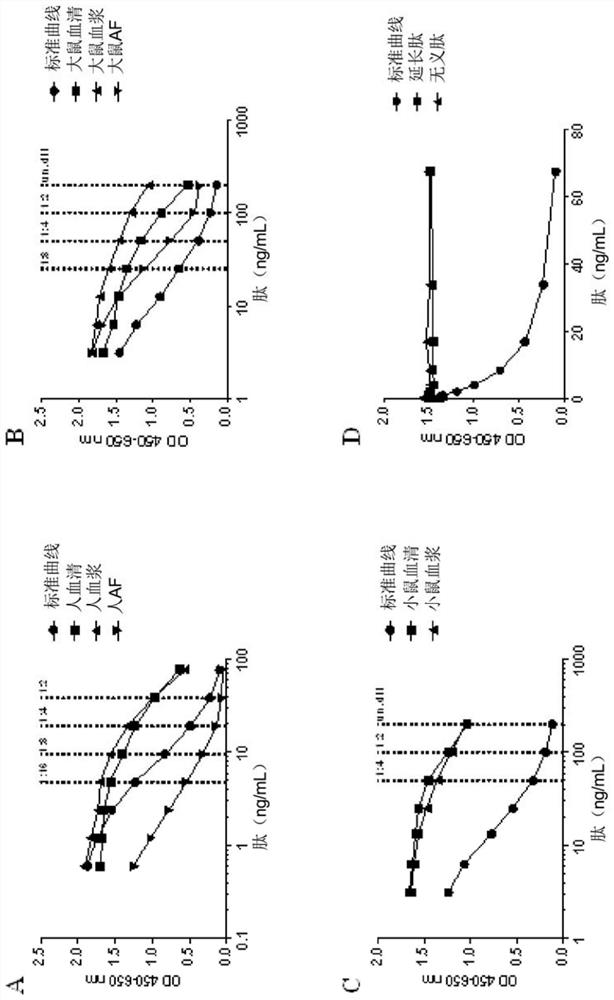
[0097] Example 3 - Determining the ratio of binding affinities
[0098] To determine the ratio of the binding affinity of the mAb to the target sequence versus the binding affinity of the mAb to the extended or shortened sequence, each sequence was synthesized and used as a calibrator peptide in the PRO-C3 ELISA as described in Example 2. The resulting calibration curve was used to determine the IC for each sequence / antibody combination 50 value. IC 50 [Target] / IC 50 The ratio of [lengthening or shortening] defines the ratio of binding affinities.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com