A kind of carbazole-based poss monomer and preparation method thereof
A technology of carbazole-based monomers, which is applied in the field of carbazole-based POSS monomers and their preparation, can solve the problems that fluorescent POSS monomers are rarely reported, and achieve improved fluorescence intensity and thermal stability, great application value, The effect of improving the adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]The 1-bromo-4(N-carbazolyl)benzene (Br-CZ) described in the examples can be prepared by referring to the literature: J.Phys.Chem.C 2009,113, 19686-19693, and the preparation steps are as follows:
[0043] (1) Under nitrogen atmosphere, 0.85g carbazole, 2.36g 1,4-dibromobenzene, 0.64g CuSO 4 and 1.38gK 2 CO 3 Add it into the reaction kettle and stir evenly, put the reaction kettle into the reaction furnace, heat to 210°C, and react for 8h.
[0044] (2) After the reaction is completed, cool to room temperature, dissolve the product in dichloromethane, filter at normal pressure, extract, dry over anhydrous magnesium sulfate, filter, rotary evaporate, use petroleum ether as eluent, separate by column chromatography, vacuum Drying afforded an off-white powdery solid.
[0045] The synthetic route is as follows:
[0046]
Embodiment 2
[0048] A kind of preparation method of carbazolyl POSS monomer, with 1-bromo-4 (N-carbazolyl) benzene (Br-CZ) and octavinyl silsesquioxane OVS as starting material, carry out Heck coupling The reaction is prepared;
[0049] The preparation steps are as follows:
[0050] (1) Under nitrogen atmosphere, add 1.8g 1-bromo-4(N-carbazolyl)benzene, 0.36g OVS, 0.05g palladium acetate, 0.14g tri-o-methylphenylphosphine, 30ml DMF, 10ml triethyl Amine, after aeration for 0.5h, heated to 100°C, reacted for 48h.
[0051] (2) After the reaction is completed, cool to room temperature, filter, wash, rotary evaporate, settle in methanol, use dichloromethane and petroleum ether (1:4) as eluent, separate by column chromatography, and dry in vacuo to obtain a light yellow powder solid , the vacuum drying temperature is 70°C, and the vacuum drying time is 24h, that is, the carbazole-based POSS monomer with fluorescence.
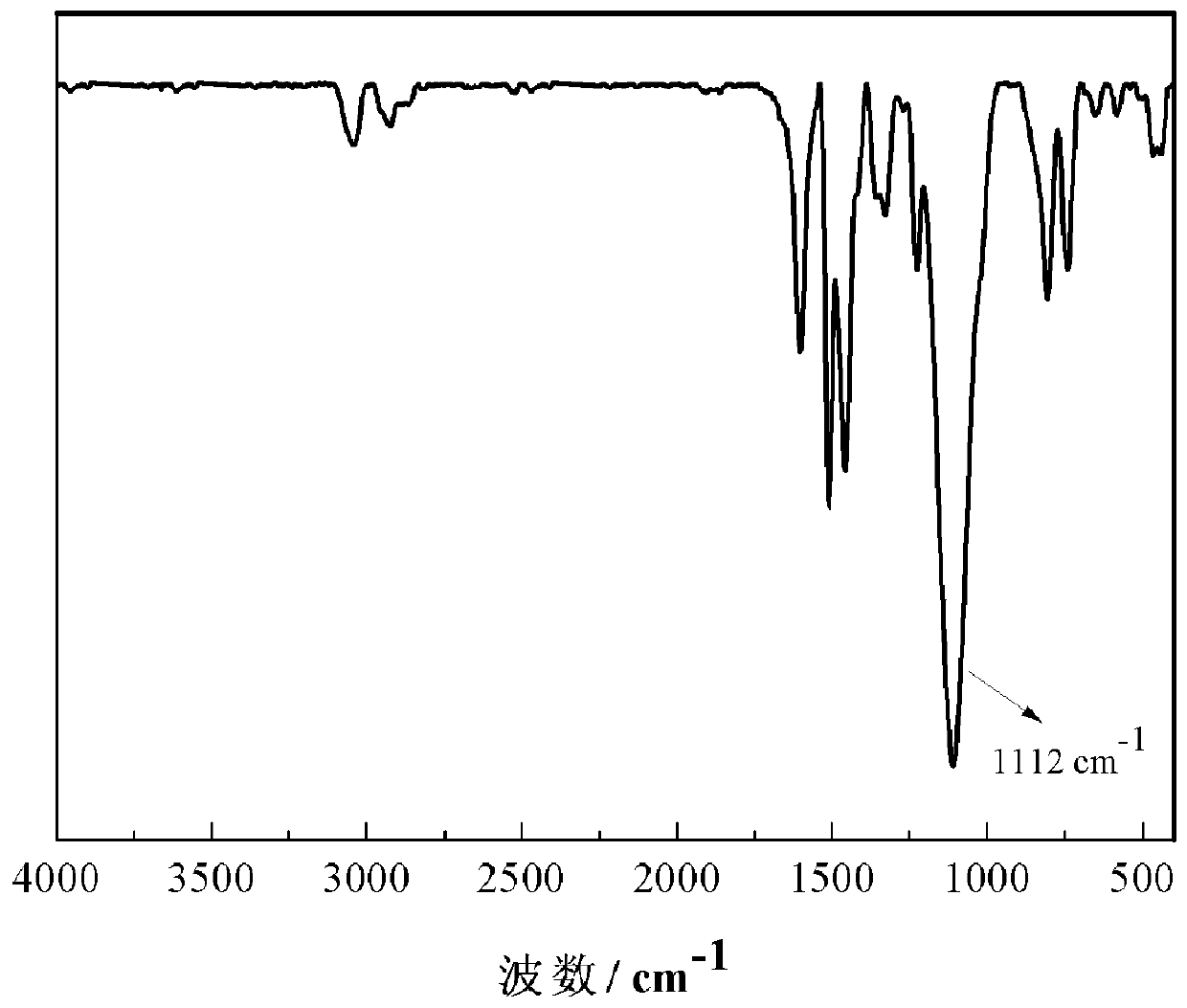
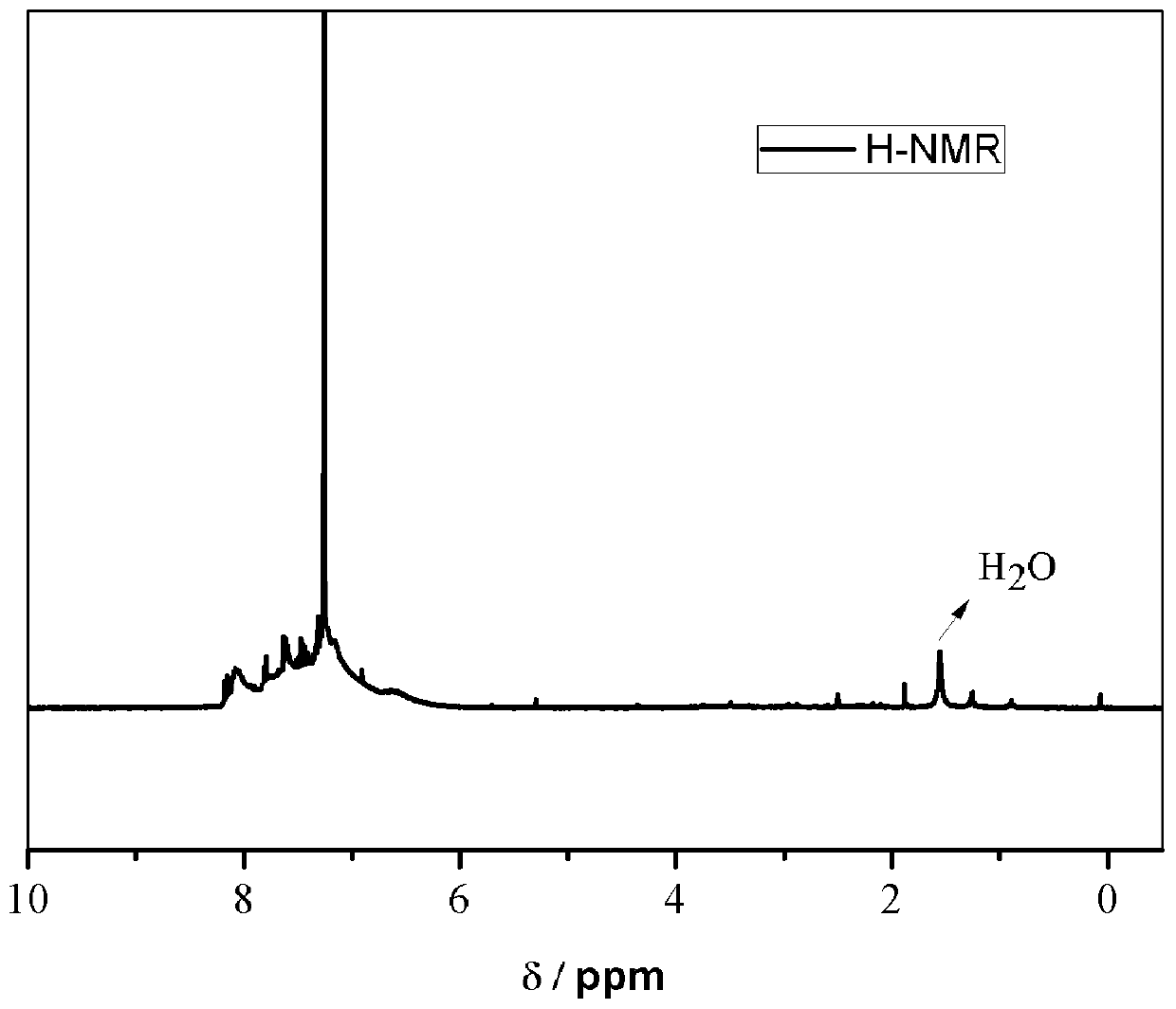
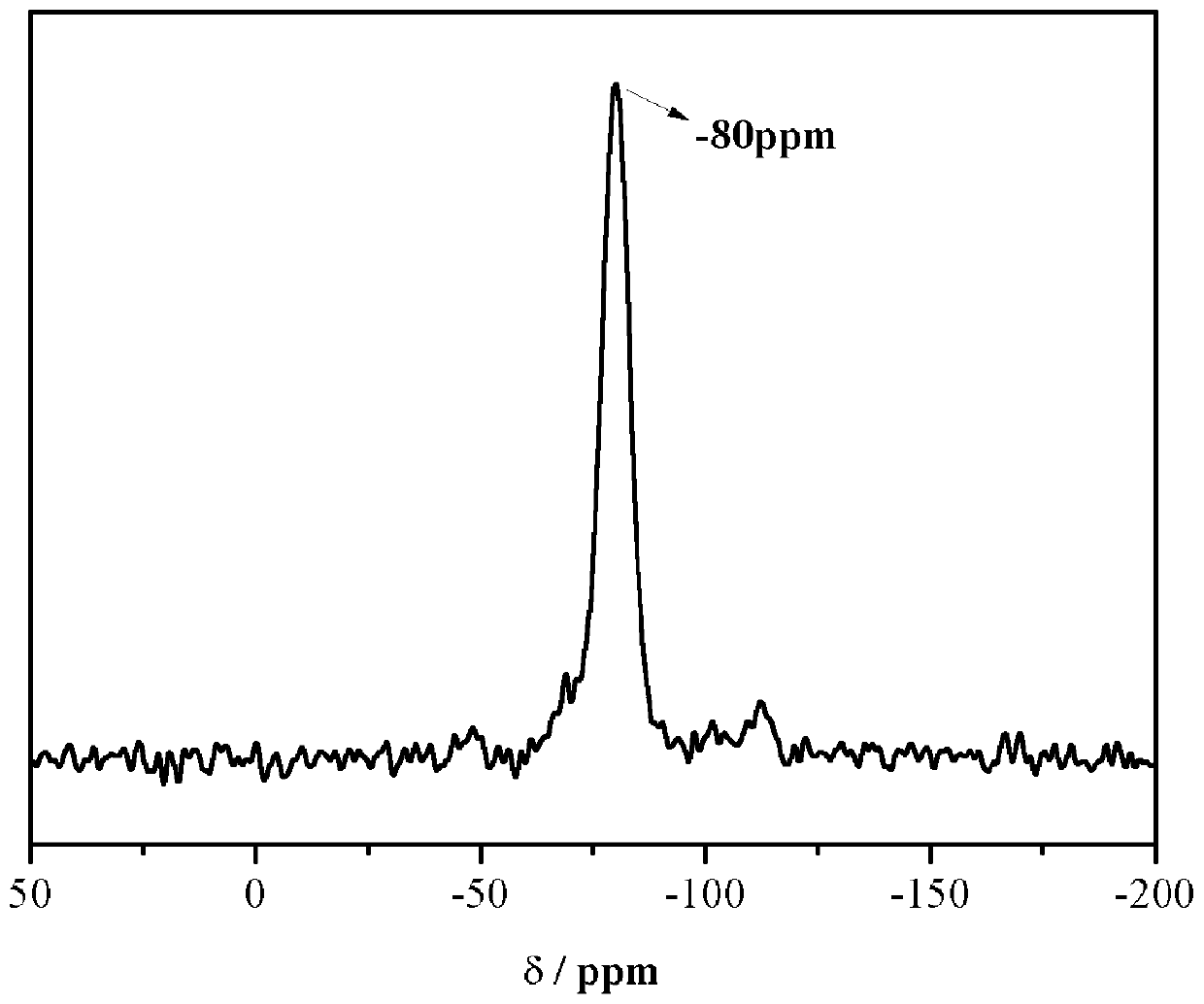
[0052] The structure of the carbazole-based POSS monomer prepared above wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com