A cationic liposome modified with hyaluronic acid, preparation method and application thereof
A technology of hyaluronic acid modification and hyaluronic acid, which is applied in the directions of liposome delivery, medical preparations of inactive ingredients, pharmaceutical formulations, etc. Low toxicity, good stability, not easy to oxidize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
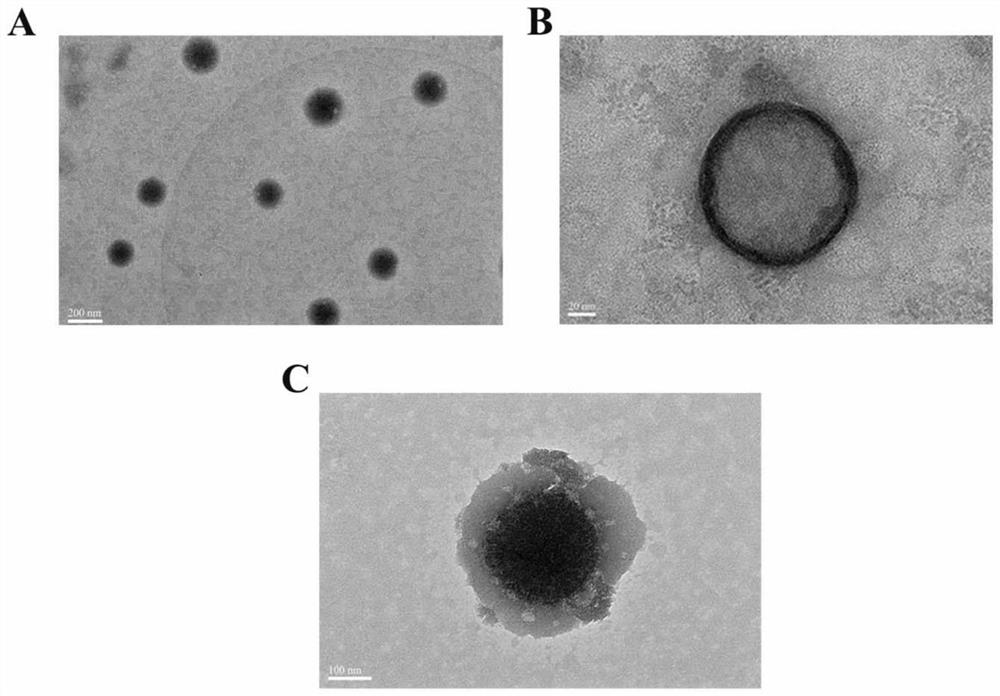
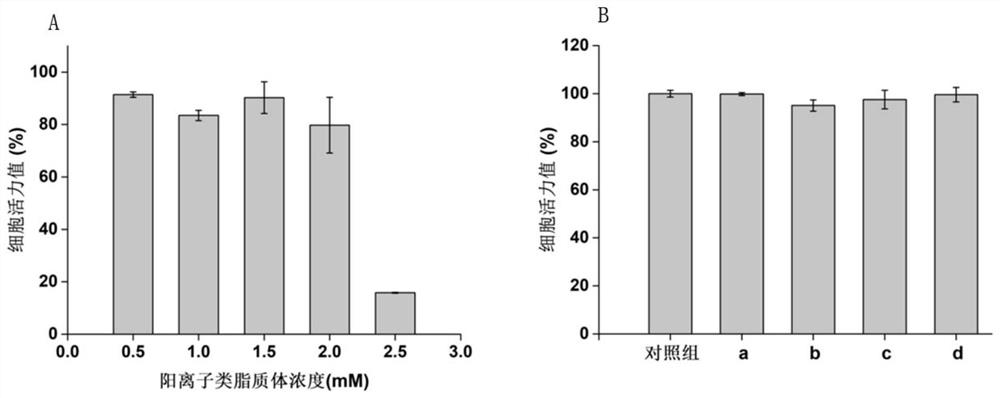
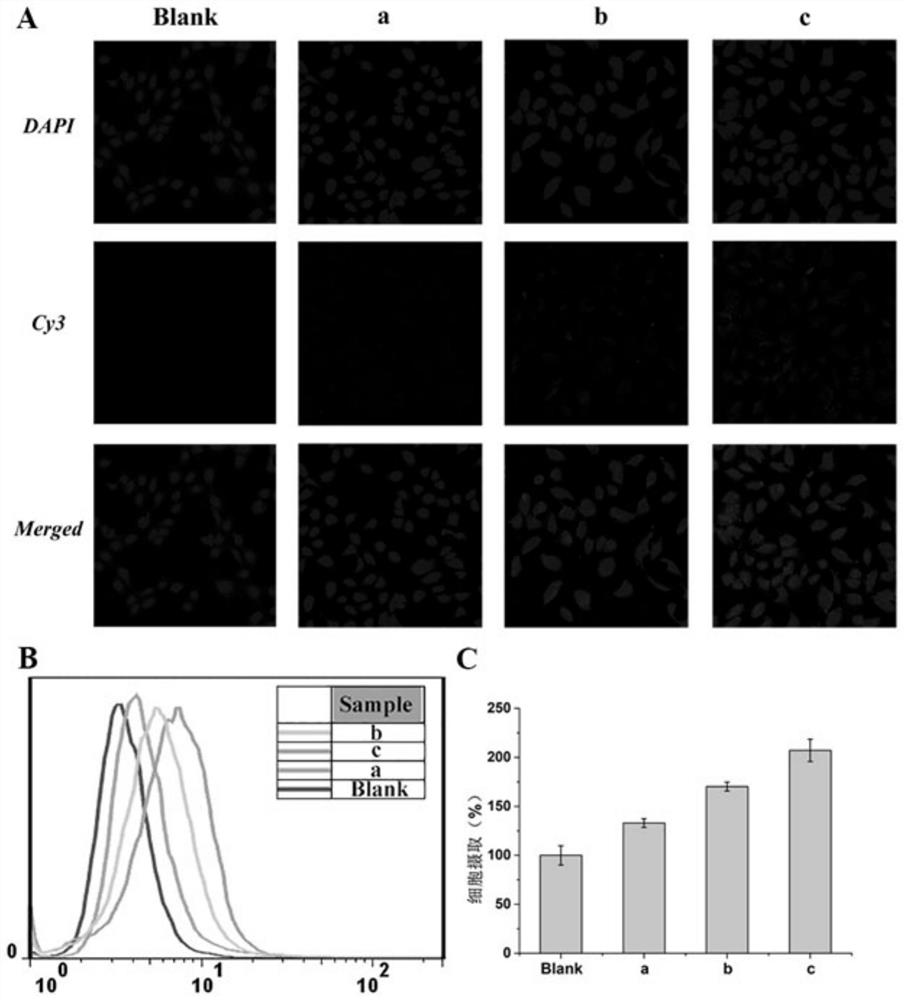
Image
Examples
Embodiment 1-5
[0029] Prescription composition: see Table 1
[0030] Preparation method: (1) mix Tween 80 and squalene in different prescription amounts, and completely dissolve them in absolute ethanol; (2) inject the ethanol solution into a buffered saline solution at a temperature of 40-80°C at a uniform speed, and Stir for 30-50nin, and then ultrafilter 1-3 times under the ultrafiltration membrane to obtain the liposome. The particle size, PDI and potential of liposomes with different molar ratios were measured with a Malvern particle size analyzer. See Table 1.
[0031] Table 1
[0032]
[0033] Compared with Example 1 and Example 2, Example 3 reduces the amount of auxiliary phospholipids used in the composition of the prescription. In comparison, the samples added with auxiliary phospholipids in Examples 1 and 2 had a cloudy appearance, while Example 3 appeared clearer with obvious blue opalescence. Compared with Example 3 and Example 4, Example 3 increases the amount of auxilia...
Embodiment 6
[0035]
[0036]
[0037] Preparation method: (1) mix the prescribed amount of Tween 80 and cholesterol, and completely dissolve it in absolute ethanol; (2) inject the ethanol solution into the buffered saline solution at a temperature of 40-80°C at a uniform speed, and stir for 30 -50nin, and then ultrafiltration under the ultrafiltration membrane for 1-3 times to obtain liposomes. The particle size, PDI and potential of the liposomes were measured with a Malvern particle size analyzer, and the results are shown in Table 2.
[0038] Table 2
[0039]
Embodiment 7
[0041]
[0042] Preparation method: (1) mix the prescribed amount of Span 80 and squalene, and completely dissolve it in absolute ethanol; (2) inject the ethanol solution into the buffered saline solution at a temperature of 40-80°C at a uniform speed, and Stir for 30-50nin, and then perform ultrafiltration under the ultrafiltration membrane for 1-3 times to obtain liposomes. The particle size, PDI and potential of the liposomes were measured with a Malvern particle size analyzer, and the results are shown in Table 3.
[0043] table 3
[0044]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com