A kind of asymmetric porphyrin organic small molecule cathode interface material and its preparation method and application
A cathode interface and small molecule technology, which is applied in the fields of organic chemistry, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problem of little research on asymmetric porphyrin cathode interface materials, so as to improve photoelectric conversion efficiency and improve Current, the effect of increasing the π-conjugate length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Synthesis of 5,15-bis(5-isooctylthiophene)porphyrin
[0042]
[0043] In a 1L three-neck round bottom flask, add 2-isooctyl-5-carbaldehydethiophene (2.24g, 10mmol), bipyrromethane (1.46g, 10mmol) and 600mL dichloromethane, exhaust with nitrogen for 30 minutes, then add 0.12mL of trifluoroacetic acid, stirred and reacted at room temperature for 12 hours, then added 1.89g of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ), continued to stir and reacted for 12 hours, and ended the reaction. Using silica gel column chromatography, dichloromethane was used as eluent, spin-dried to obtain the crude product, and then recrystallized with chloroform / methanol to obtain a dark red solid. The NMR data of the red solid are: 1 H NMR (500MHz, Chloroform-d) δ / ppm 10.28(s, 2H), 9.38(d, J=4.6Hz, 4H), 9.35(d, J=4.6Hz, 4H), 7.74(d, J=3.3 Hz,2H),7.22–7.20(m,2H),3.12–3.09(m,4H),1.38–1.18(m,18H),1.08(t,J=7.4Hz,6H),1.01(t,J= 7.1Hz, 6H), -2.96(s, 2H).
[0044] (2) Synthesis of 5-bromo-...
Embodiment 2
[0063] 5-triisopropylsilylacetylene-15-(9,9-bis(3'-(N,N-dimethyl-N-ethylammonium bromide)propyl)fluorene-2-acetylene)-10, Synthesis of 20-bis(5-isooctylthiophene) zinc porphyrin
[0064]
[0065] Add 5-triisopropylsilylacetylene-15-(9,9-bis(3'-(N,N-dimethylamino)propyl)fluorene-2-acetylene)-10,20 into a 50mL two-necked flask - Bis(5-isooctylthiophene) zinc porphyrin (50mg, 0.038mmol) and N,N-dimethylformamide 1ml and 1ml tetrahydrofuran, stir to dissolve and add bromoethane 1ml, heat to 60°C and keep it The temperature was reacted for 12 hours, the reaction was completed, and the product was obtained by centrifugation. Mass(MALDI-TOF): Obs.1517.8; Calcd.for C 84 h 108 N 6 Br 2 S 2 SiZn, 1519.2.
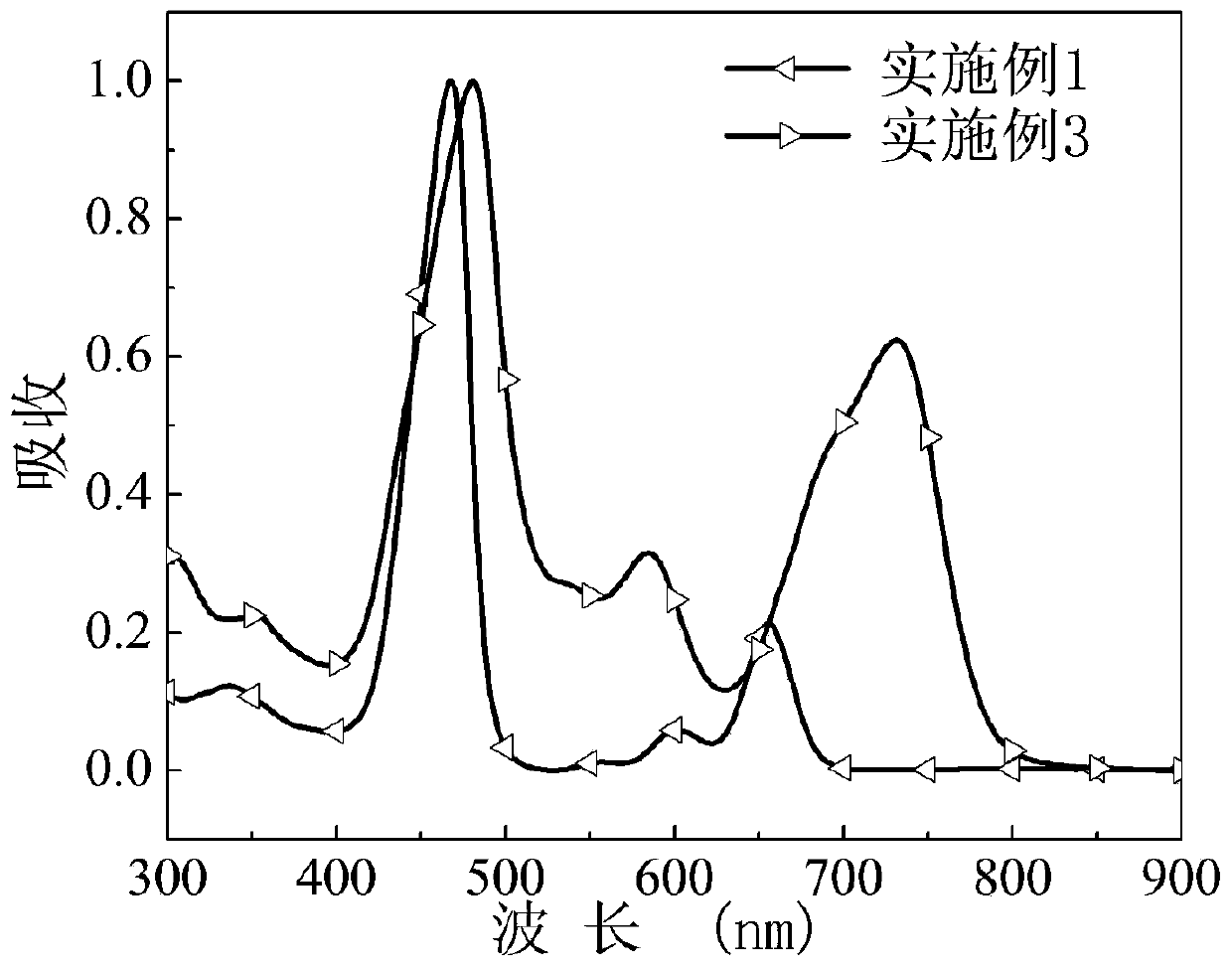
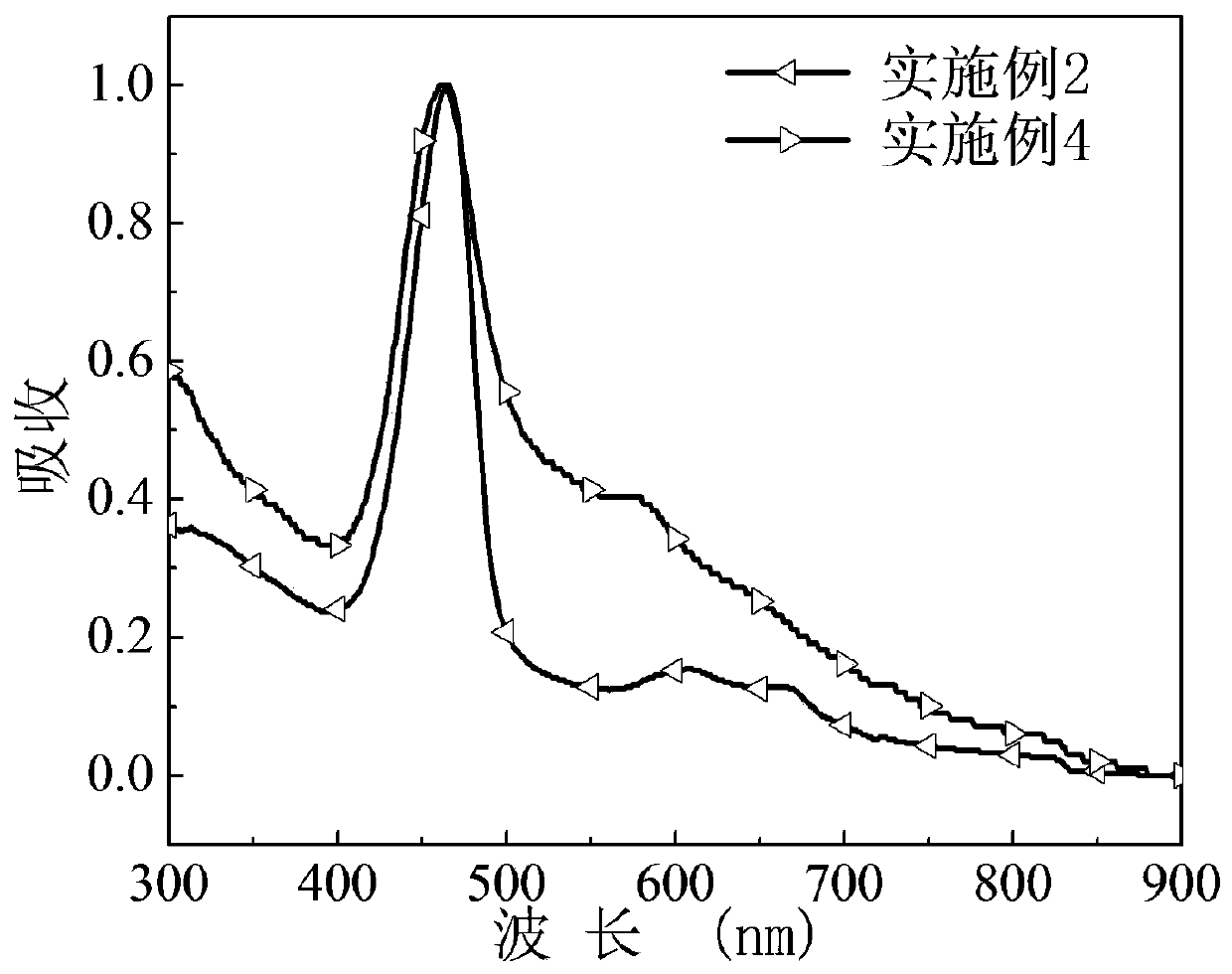
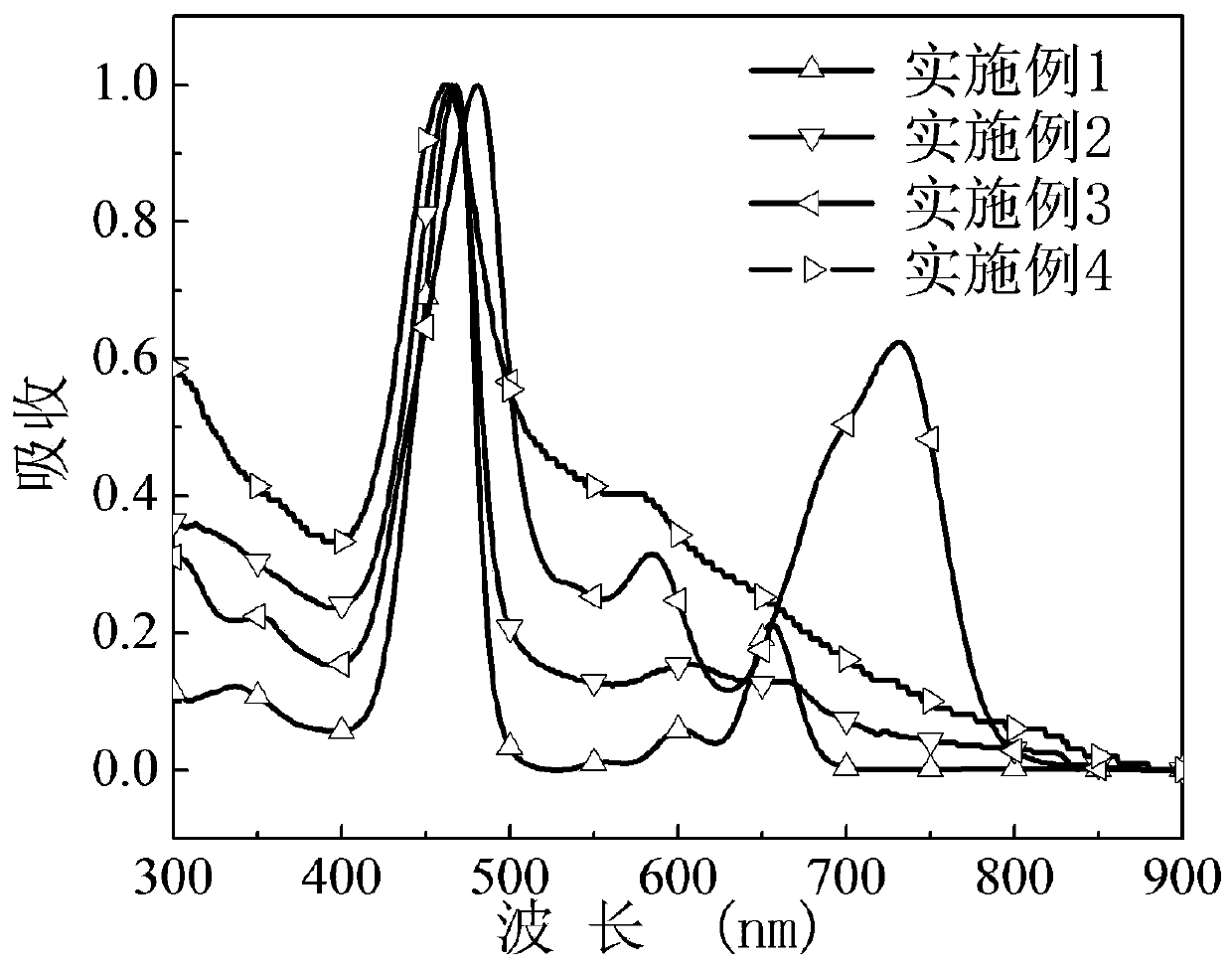
[0066] The asymmetric porphyrin organic small molecule cathode interface material prepared in this example was dissolved in methanol, and the ultraviolet-visible absorption spectrum of the obtained methanol dilute solution was as follows: figure 2 .
[0067] The asymmetri...
Embodiment 3
[0070] (1) 5-ethyne-15-(9,9-bis(3'-(N,N-dimethylamino)propyl)fluorene-2-ethyne)-10,20-bis(5-isooctyl Synthesis of thiophene) zinc porphyrin
[0071]
[0072] Add 5-triisopropylsilylacetylene-15-(9,9-bis(3'-(N,N-dimethylamino)propyl)fluorene-2-acetylene)-10,20 to a 100mL one-necked flask - Bis(5-isooctylthiophene) zinc porphyrin (91mg, 0.07mmol) and 20ml tetrahydrofuran were stirred and dissolved, and 0.07ml of 1mol / L tetrabutylammonium fluoride (TBAF) tetrahydrofuran solution was added, and stirred at room temperature for 5min, After the reaction was completed, it was extracted with chloroform and spin-dried to obtain the product.
[0073] (2) 5-(9,9-bis(3'-(N,N-dimethylamino)propyl)fluorene-2-ethyne)-15-(3-(2-thiophene)-2,5 -Bis(2-ethylhexyl)-6-(2-thiophene)pyrrole[3,4-c]pyrrole-1,4(2H,5H)-dione)-10,20-bis(5-isooctyl Synthesis of thiophene) zinc porphyrin
[0074]
[0075]5-ethyne-15-(9,9-bis(3'-(N,N-dimethylamino)propyl)fluorene-2-ethyne)-10,20-bis(5-isooctylthioph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com