Application of trialdehyde phloroglucinol thiosemicarbazone hybrid compound in antineoplastic drugs
A technology for reducing amino groups of trialdehyde group phloroglucinol and amino reduction technology of aldehyde group phloroglucinol, which is applied in the field of trialdehyde group phloroglucinol thiosemicarbazones, and can solve the problem of amino reduction without trialdehyde group phloroglucinol Reported issues such as thiourea, to achieve the effects of poor curative effect, short survival period, and rapid disease progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis and structure identification of embodiment 1 compound
[0026] The preparation steps of 2,2',2"-((2,4,6-trihydroxyphenyl-1,3,5-triyl)tri(methylidene))tri(thiosemicarbazide) of the present invention are as follows: Weigh 10mmol2,4,6-triformylpyroglucinol and 33mmol thiosemicarbazide (6.485g) into a reaction flask, then add 50mL of ethanol (95%) and stir evenly, then add 3mL of glacial acetic acid dropwise, and then 65- Reflux and stir at 70°C for about 10 hours, evaporate most of the ethanol under reduced pressure, add 20 ml of ice water, filter to obtain a precipitate, wash the precipitate with ice water (30 mL for three times), and recrystallize from ethanol to obtain compound I. 1 H NMR (DMSO-d 6 ,500MHz,ppm):δ11.50(broad,NH),11.10(s,NH),8.56(s,3H),8.06(broad,OH); 13 C NMR (DMSO-d 6 ,125MHz,ppm):δ177.4(3C),160.0(3C),142.3(3C),99.6(3C)
Embodiment 2
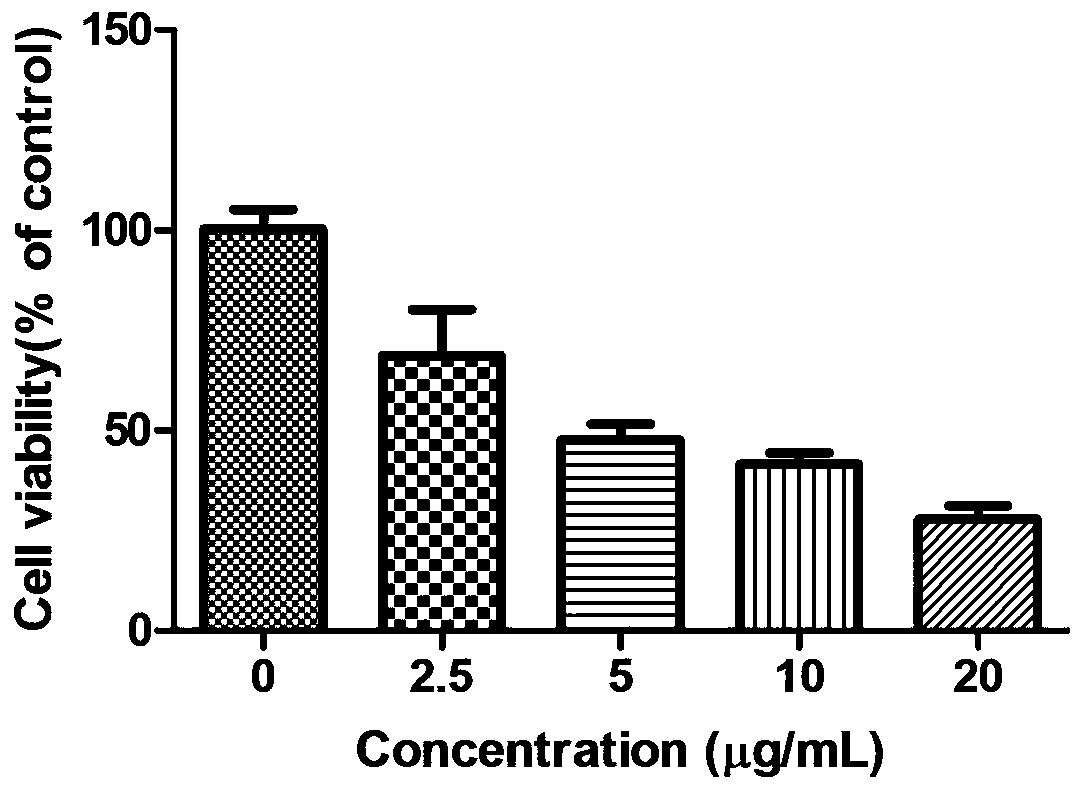
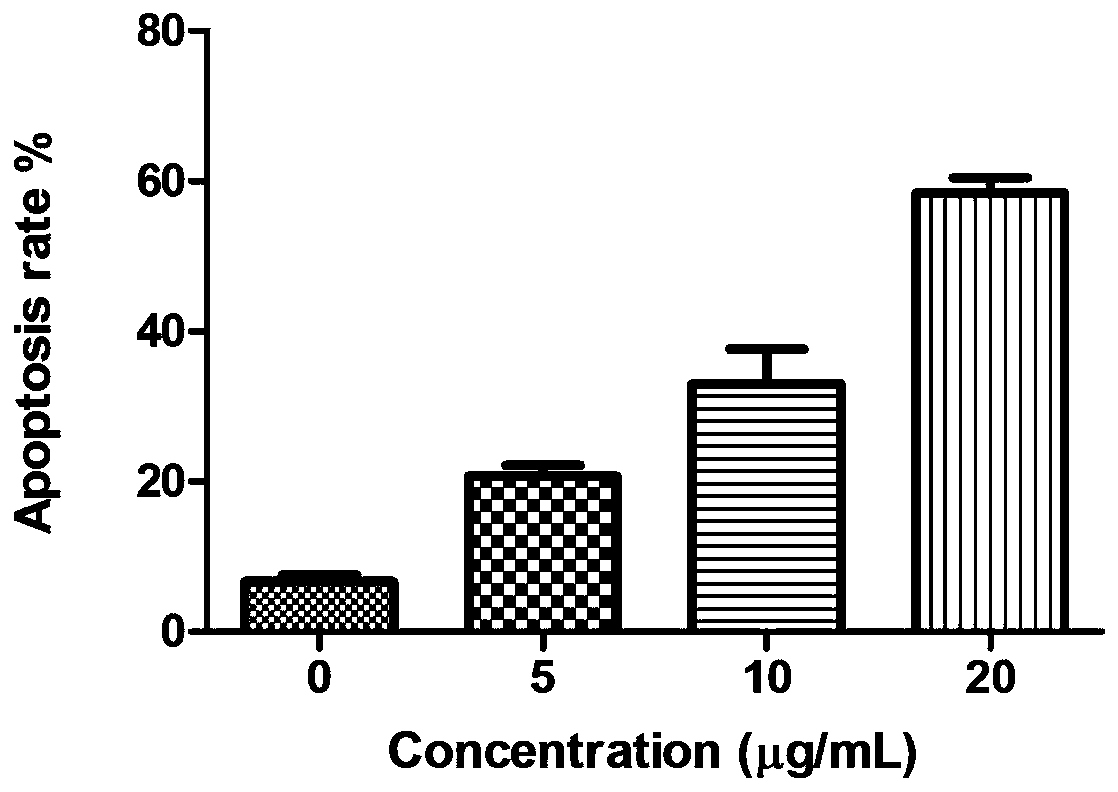
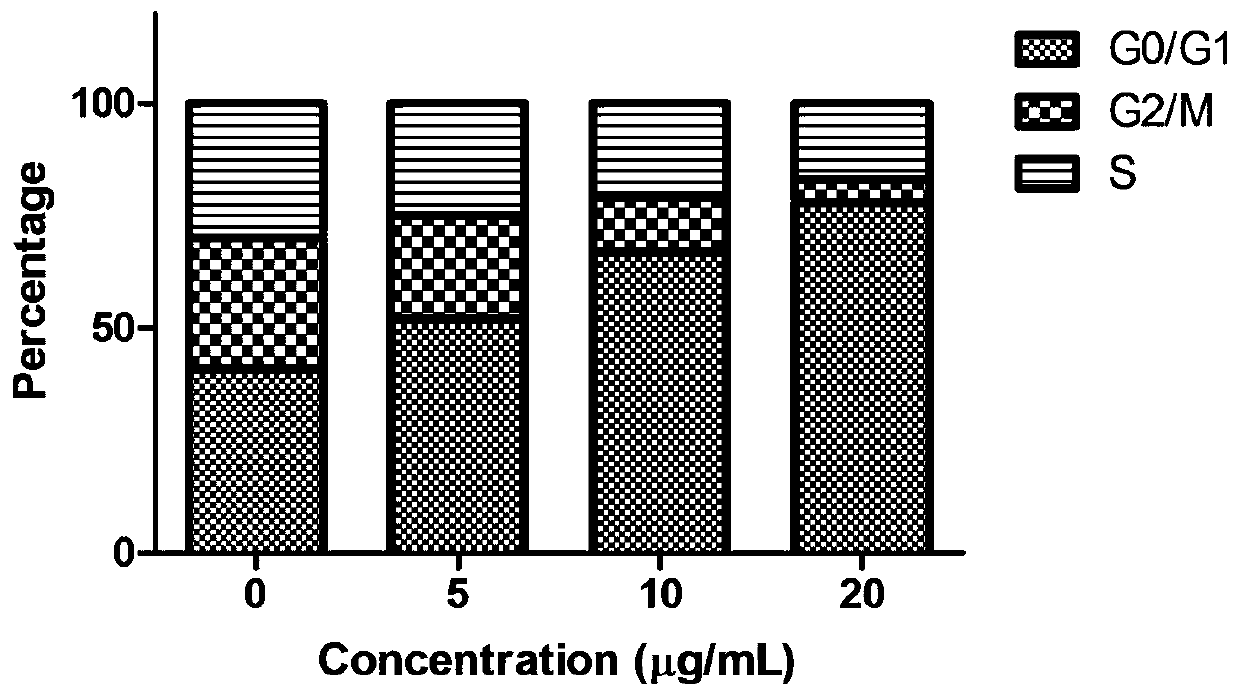
[0027] Example 2 2,2',2"-((2,4,6-trihydroxyphenyl-1,3,5-triyl)tri(methylidene))tri(thiosemicarbazide)(I) Study on the proliferative activity of HepG2 cells
[0028] 1. Experimental drugs
[0029] 2,2',2"-((2,4,6-trihydroxyphenyl-1,3,5-triyl)tri(methylidene))tri(thiosemicarbazide)(I) was synthesized by our laboratory It is prepared by using DMSO to aid dissolution, and configured as a 20mg / ml storage solution for long-term storage. The maximum concentration for the experiment is 5, 10, and 20 μg / ml.
[0030] 2. Cell lines
[0031] Human hepatoma cells (HepG2) were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. Use DMEM medium containing 10% peptide bovine serum at 37°C with a volume fraction of 5% CO 2 1. Routine culture in the air under fully saturated humidity conditions, replace the medium after 48 hours, when the cell growth reaches saturation, digest with 0.25% trypsin and passage once every 2-3 days, and use logarithmic growth phase cells in...
Embodiment 3
[0036] Example 3 2,2',2"-((2,4,6-trihydroxyphenyl-1,3,5-triyl)tri(methylidene))tri(thiosemicarbazide)(I) Study on the clone formation of HepG2
[0037] 1. Experimental cells:
[0038] Same as Example 1
[0039] 2. Experimental drugs:
[0040] Same as Example 1
[0041] 3. Experimental method:
[0042] Take the logarithmic phase liver cancer HepG2 cells, trypsinize and resuspend, inoculate in a six-well plate, inoculate 200 cells per well, and store at 37°C, 5% CO 2 After culturing in the incubator for 24 hours, different concentrations of compound (I) (5, 10, 20 μg / mL) were added for 10 days, and the medium was replaced every 2 days, and a blank group (DMSO) was set at the same time. After the experiment, discard the medium, fix the cells with 4% paraformaldehyde for 15 min, wash the cells with PBS 3 times, add 1 mL of crystal violet with a mass fraction of 0.2% to each well, act for 15 min, wash the cells with PBS 3 times After the culture plate is dried, take pictures ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com