Application of phenylacrylic compounds to preparation of Shp2-specific inhibitors and pharmaceutical application thereof
A technology of phenylacrylic acid compounds, which is applied in the field of preparing Shp2 specific inhibitors of phenylacrylic acid compounds, and can solve problems such as few and difficult development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
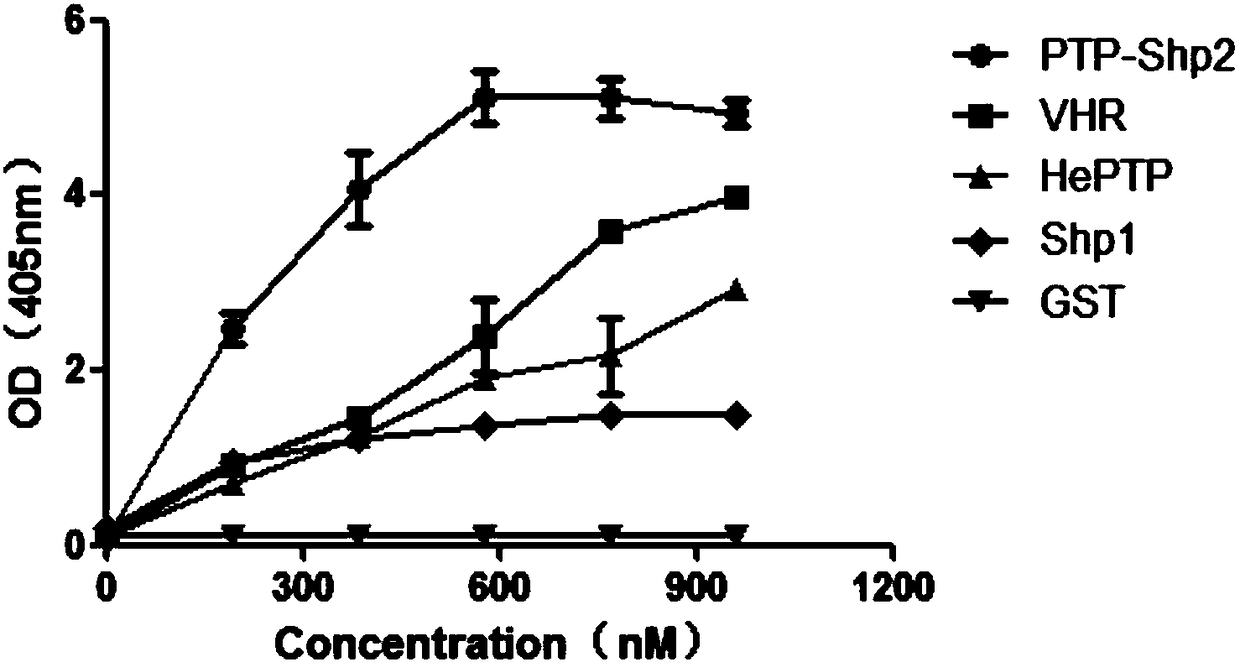
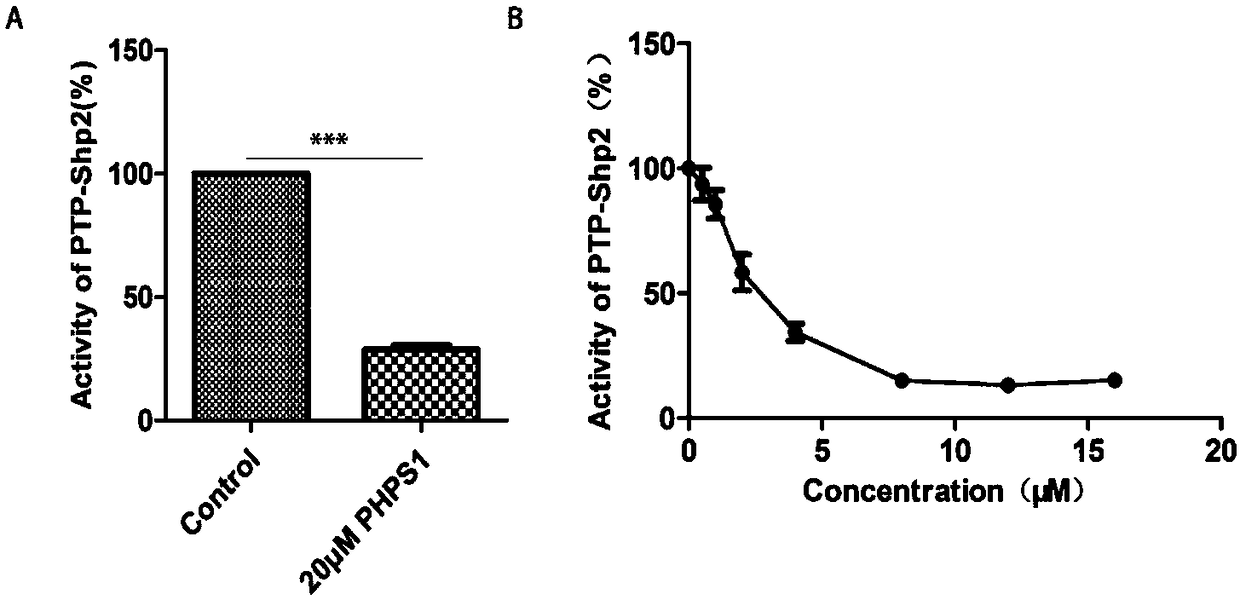
[0039] Example 1: Establishment of a model for high-throughput screening of PTP-Shp2 protein inhibitors in vitro
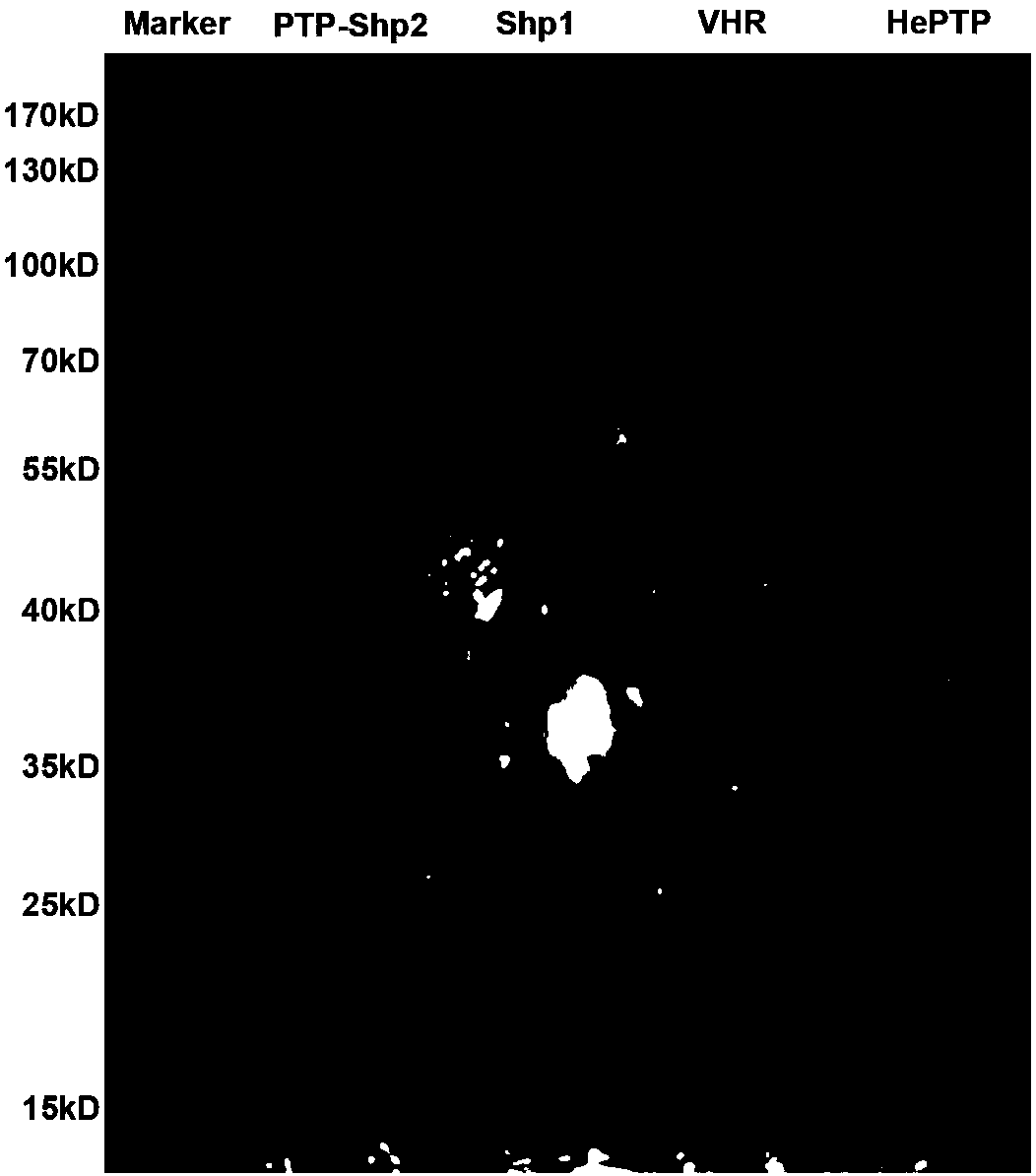
[0040] 1) Prokaryotic expression and purification of PTP-Shp2, Shp1, VHR, HePTP proteins
[0041] After pGEX4T-1-PTP-Shp2, pGEX4T-1-Shp1, pGEX4T-1-VHR, pGEX4T-1-HePTP plasmids (provided by the laboratory of Professor Feng Gensheng, University of California, San Diego) transformed into BL21Star (DE3) competent cells, 37 Cultivate overnight in an incubator at ℃, pick a single colony and shake it in LB medium to obtain a large amount of bacterial liquid. A large amount of bacterial liquid expressing PTP-Shp2 protein was at 20°C, and a large amount of bacterial liquid expressing Shp1, VHR, HePTP protein was induced overnight at 0.1mMIPTG160r / min at 25°C, and the bacterial cells were collected by centrifugation, resuspended and ultrasonically disrupted And purification, and then use SDS-PAGE electrophoresis to verify the purification effect. PTP-Shp2, Shp1, VHR, and ...
Embodiment 2
[0056] Example 2: Effect of H320 (isoferulic acid) on the activation of Shp2-dependent Erk induced by EGF
[0057]Previous studies have shown that Shp2 can participate in the regulation of the Ras / Erk signaling pathway. In cells, the growth factor EGF can cause the cascade reaction of the Erk pathway in a Shp2-dependent manner, thereby causing an increase in the phosphorylation level of Erk. Therefore, we tested at the cellular level whether the PTP-Shp2 inhibitor H320 (isoferulic acid) obtained by our high-throughput screening could affect EGF-induced Erk phosphorylation by inhibiting the activity of Shp2. 293T cells after overnight serum starvation were treated with different concentrations of H320 (isoferulic acid) (0 μM, 10 μM, 20 μM, 30 μM) for 24 h, stimulated with EGF (2 ng / ml) for 10 min, and then added a certain volume of RIPA lysate, Proteins were harvested and Westernized. from Figure 5 It can be seen that, under no drug treatment, the level of p-Erk was signific...
Embodiment 3
[0058] Example 3: Effect of H335 (cinnamic acid) on EGF-induced activation of Shp2-dependent Erk
[0059] Previous studies have found that Shp2 is necessary for the maintenance of related cellular functions in breast cancer MDA-MB-468 cells. We detected the effect of H335 (cinnamic acid) on the Ras / Erk signaling pathway regulated by Shp2 in MDA-MB-468 cells. MDA-MB-468 cells were starved for 16 h, treated with different concentrations of H335 (cinnamic acid) (0 μM, 10 μM, 20 μM) for 3 h, induced with 2 ng / ml EGF for 10 min, and the expression levels of p-Erk, Erk, Shp2, and GAPDH were detected by Western analysis. Image 6 It shows that adding different concentrations of H335 (cinnamic acid) to treat MDA-MB-468 cells can significantly reduce the expression level of p-Erk induced by EGF. Next, we also tested whether the inhibitory effect of H335 (cinnamic acid) on Shp2 is time-sustained, and found that p-Erk was not up-regulated at different times of EGF induction, indicating ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com