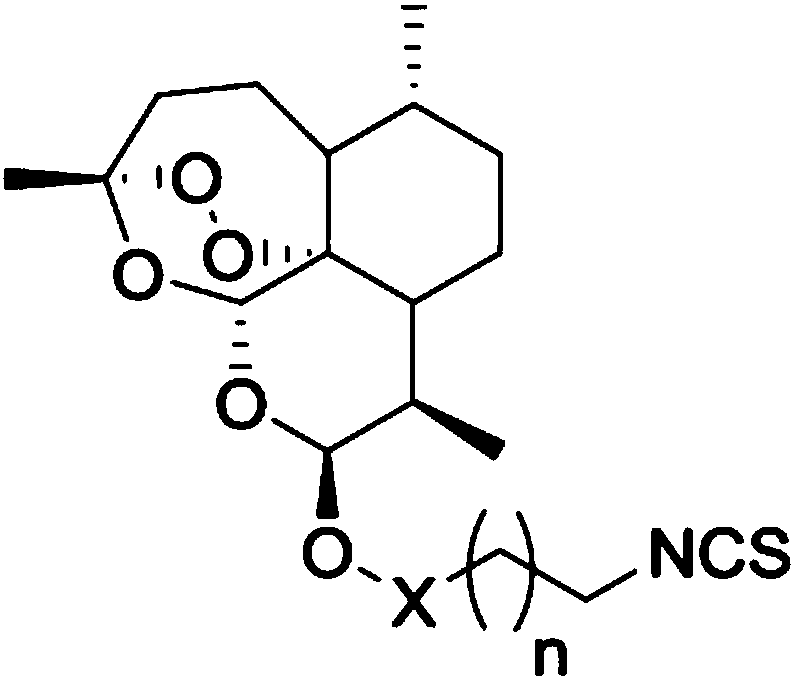
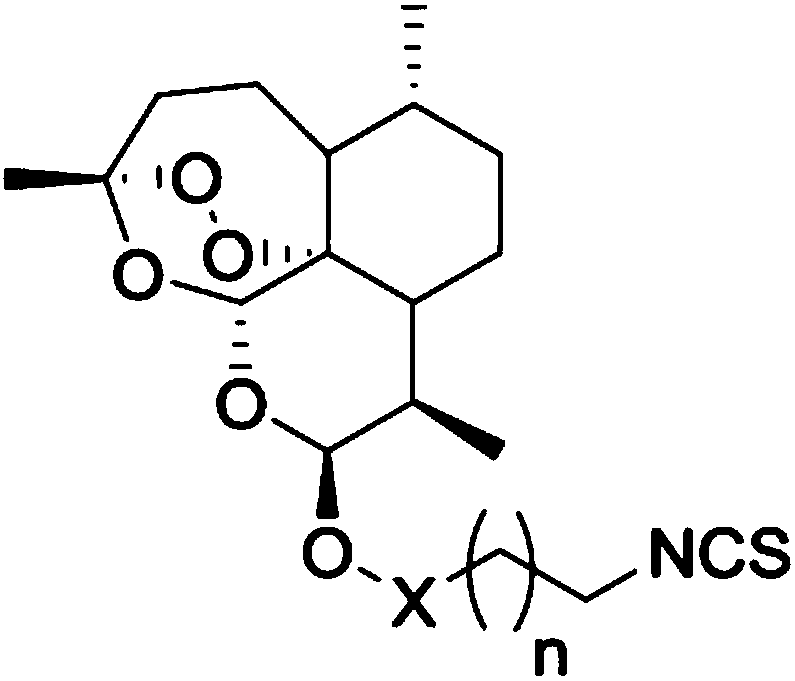
Artemisinin derivatives containing isothiocyanate group and application thereof
A technology of artemisinin derivatives and isothiocyanic acid, which is applied in the field of medicine, can solve problems such as difficult radical cures, and achieve high cytotoxicity and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
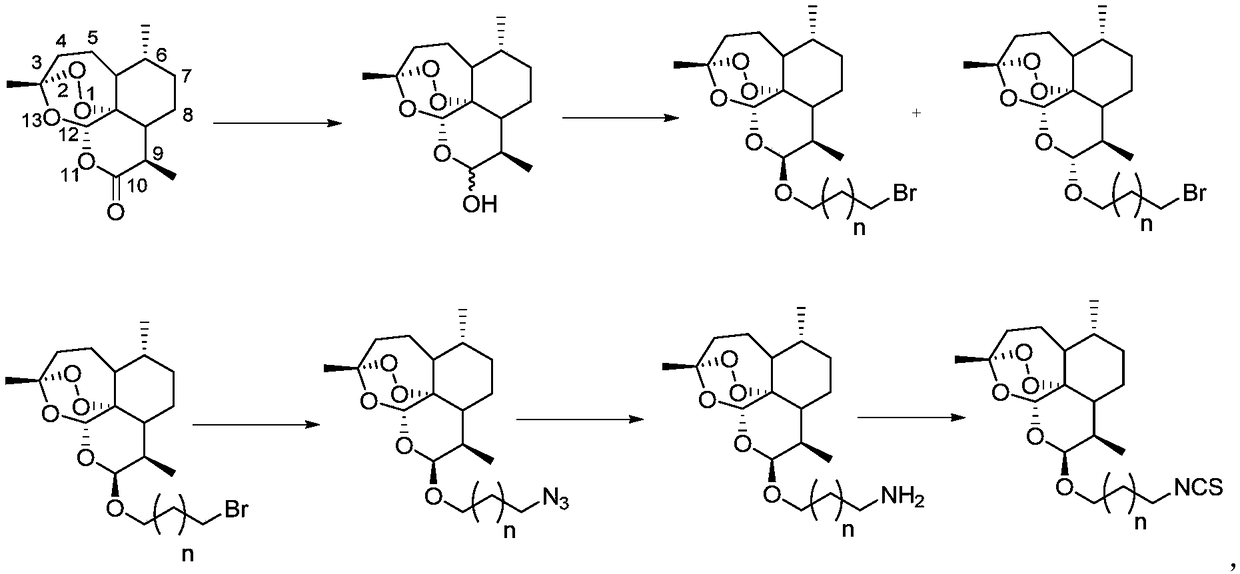
[0031] The present invention also provides a preparation method of the above-mentioned compound of formula I.
[0032] The embodiments of the present invention specifically describe three embodiments of the compound of formula I above: X is methylene, and n=0, n=1 or n=4.
[0033] Please see the following synthetic routes:
[0034]
Embodiment 1
[0036] Example 1 provides the preparation method of 5a in the above synthetic route, where X in formula I is methylene, and n=0.
[0037] details as follows:
[0038] Artemisinin (ART) was purchased from Aladdin Reagent Company.
[0039] Dihydroartemisinin (DHA) synthesis: Artemisinin (5.0 g, 18 mmol) was dissolved in anhydrous methanol (120 mL), placed in an ice-water bath, and NaBH was added in batches under stirring 4 (1.0g, 27mmol), the addition was completed within 20min. After the reaction was completed for about 2 hours, adjust the pH to 6-7 with glacial acetic acid, evaporate most of the methanol, add water and stir for 15 minutes, filter, collect the precipitate, wash with water three times, and dry to obtain a white solid (3.0 g, 59%). 1 H NMR (400MHz, CDCl 3 )δ5.59(s,1H),5.36(s,1H),5.29(s,1H),4.72(t,J=8.8Hz,1H),3.45(d,J=8.6Hz,1H),3.19( s,1H),2.57(d,J=3.4Hz,1H),2.41-2.23(m,3H),2.06-1.96(m,2H),1.40(s,3H),1.39(s,3H),0.93 (s,6H),0.92(s,3H),0.91(s,3H); 13 CNMR (100...
Embodiment 2
[0049] Example 2 provides the preparation method of 5b in the above synthetic route, X in formula I is methylene, and n=1.
[0050] In this example, the synthesis of dihydroartemisinin (DHA) is the same as that in Example 1, and will not be repeated in this example.
[0051] Synthesis of compound (1b): Dihydroartemisinin (840mg, 3.0mmol) and 3-bromo-1-propanol (0.42mL, 4.6mmol) were dissolved in CH at below 0°C 2 Cl 2 (20mL), add BF dropwise 3 ·Et 2 O (0.63mL, 5.0mmol), continued to stir at the same temperature for 5min after the dropwise addition was completed, then transferred to an ice-water bath, and continued to stir for about 50min. After the reaction was completed, the saturated NaHCO 3 Solution, water and saturated brine were washed, the organic layer was collected, dried over anhydrous sodium sulfate, and concentrated to obtain a light yellow oily crude product. The crude product was separated by silica gel chromatography (ethyl acetate:petroleum ether=3:97) to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com