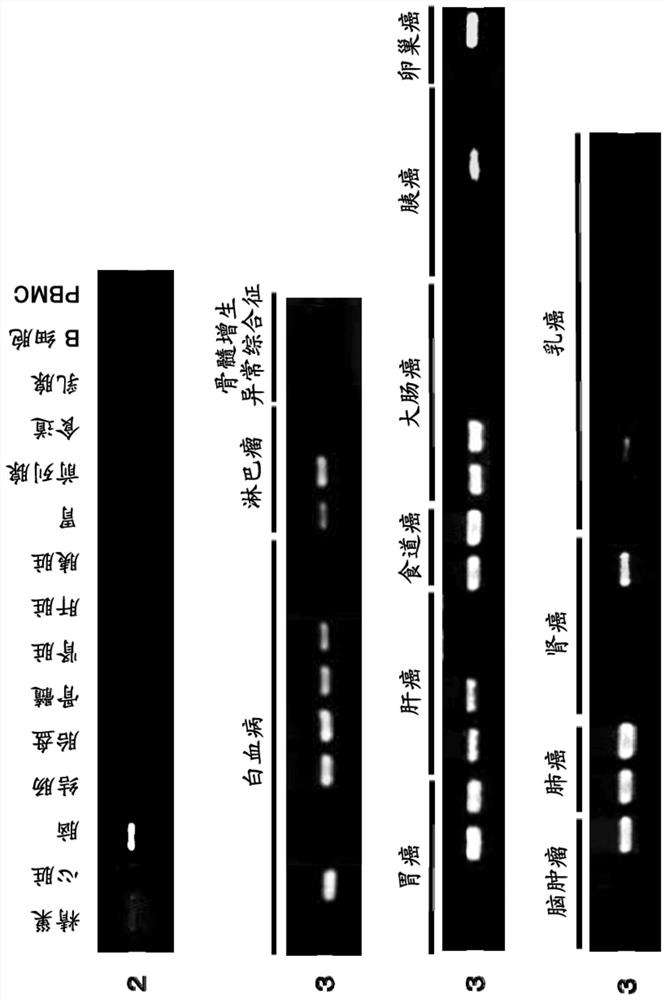
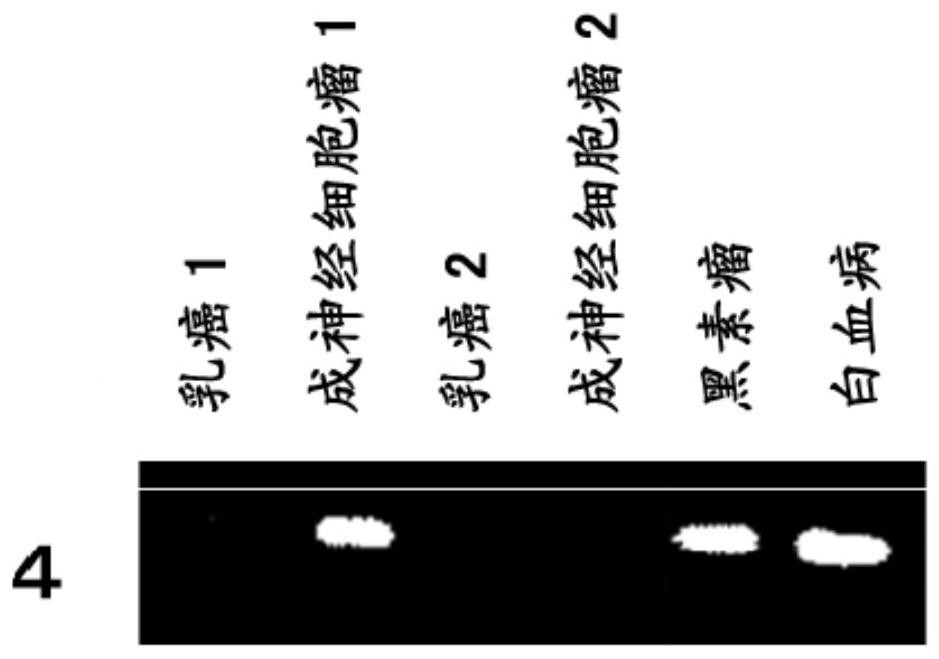
immune inducer
An immune inducer, amino acid technology, used in vaccines, lung cancer vaccines, kidney cancer vaccines, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] (Example 1) Obtaining novel cancer antigen protein by SEREX method
[0104] (1) Preparation of cDNA library
[0105] Total RNA was extracted from canine testis by the acid guanidium-phenol-chloroform method (Acid guanidium-Phenol-Chloroform method), and polyadenosine was purified using Oligotex-dT30 mRNA furification Kit (manufactured by Takara Shuzo Co., Ltd.) according to the protocol attached to the kit acid RNA.
[0106] A cDNA phage library was synthesized using the obtained mRNA (5 μg). For the preparation of the cDNA phage library, cDNA Synthesis Kit, Zap-cDNA Synthesis Kit, and ZAP-cDNA GigapackIII Gold Cloning Kit (manufactured by STRATAGENE) were used, and the library was prepared according to the protocol attached to the kit. The size of the prepared cDNA phage library is 1×10 6 pfu / ml.
[0107] (2) Screening cDNA library by serum
[0108] Immunoscreening was performed using the cDNA phage library prepared above. Specifically, the host Escherichia coli ...
Embodiment 2
[0116] (Example 2) Analysis of cancer antigenicity of MRAP2 in vivo
[0117] (1) Production of recombinant vector expressing mouse MRAP2 in vivo
[0118] Based on the nucleotide sequence of SEQ ID NO: 7, a recombinant vector expressing mouse MRAP2 in vivo was prepared by the following method. PCR was performed with 1 μl of cDNA prepared from the mouse leukemia cell line EL4 (purchased from ATCC) whose expression was observed in Example 1, and two primers (described in SEQ ID NOs: 17 and 18) containing EcoRI and NotI restriction sequences. 0.4 μM, 0.2 mM dNTP, 1.25 U each of PrimeSTAR HS polymerase (manufactured by Takara Shuzo Co., Ltd.) were added to make the total amount of each reagent and buffer solution 50 μl, and a thermal cycler (manufactured by BIO RAD) was used to heat the mixture at 98° C. for 10 The cycle of seconds, 55°C for 15 seconds, and 72°C for 1 minute was repeated 30 times. In addition, the above-mentioned two kinds of primers are primers for amplifying th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com