Therapeutic for gout or hyperuricemia
A technology for hyperuricemia and gout, which can be used in drug combination, drug delivery, pharmaceutical formulation and other directions, and can solve problems such as the use of colchicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0255] Hereinafter, the present invention will be described in more detail with examples, reference examples, comparative reference examples, test examples, etc., but the present invention is not limited to these contents.
[0256]
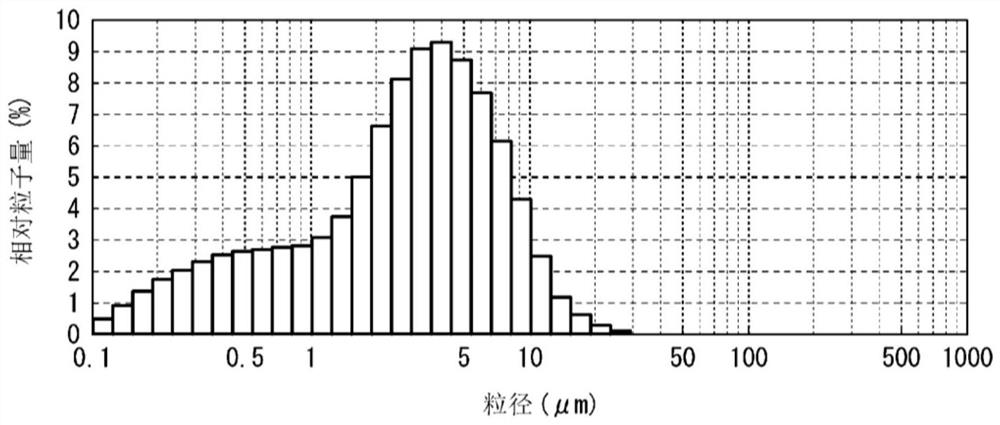
[0257] Dissolve 9 g of compound 14 (2-(3-cyano-4-phenoxyphenyl)-7-hydroxythiazolo[5,4-d]pyrimidine) in tetrahydrofuran (sometimes abbreviated as THF), ethanol and water After 1936g of mixed solvent (weight ratio: THF / ethanol / water=1600.5 / 254.5 / 81) (slightly heated and dissolved), it is pumped into the spray drier via a peristaltic pump at a speed of about 5mL / min, and is injected from a 2-fluid nozzle (diameter 508μm) with an inlet temperature of 80°C, an outlet temperature of about 60°C, and a dry air volume of 0.30m 3 / min and nozzle spray air pressure 1.0kgf / cm 2 conditions for spray drying and granulation. The obtained dried product was left overnight at room temperature to obtain 100% amorphous compound 14.
[0258] For the volume averag...
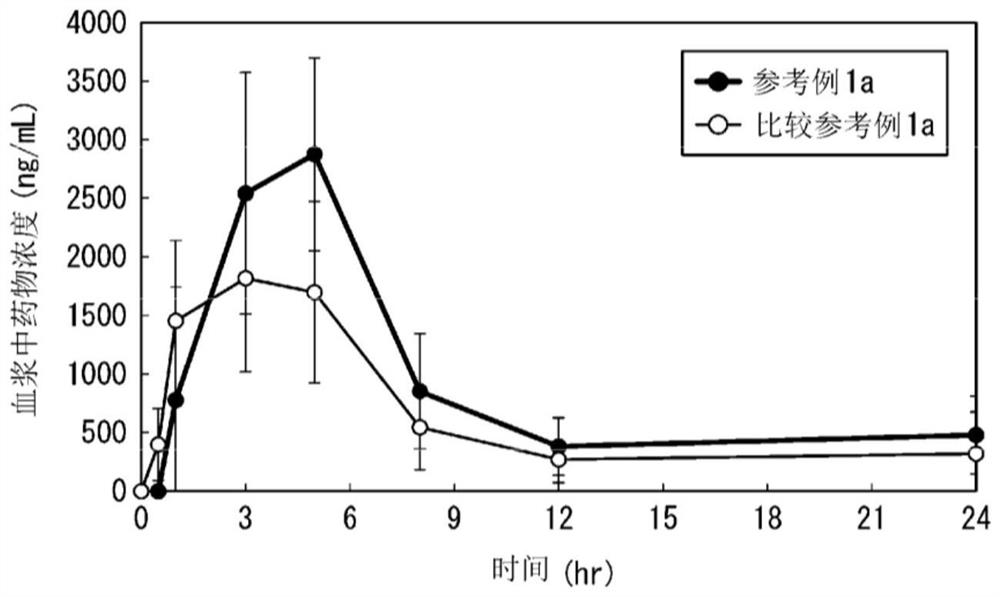
reference example 1
[0271] The HPMCAS used in Reference Example 1b is MF type (that is, the substitution ratio per 1 monomer unit is methoxy: 21.0-25.0%, hydroxypropoxy: 5.0-9.0%, acetyl: 7.0-11.0%, amber Acyl: 10.0-14.0%, viscosity: 2.4-3.6mPa·s).
[0272] [Form 2B]
[0273]
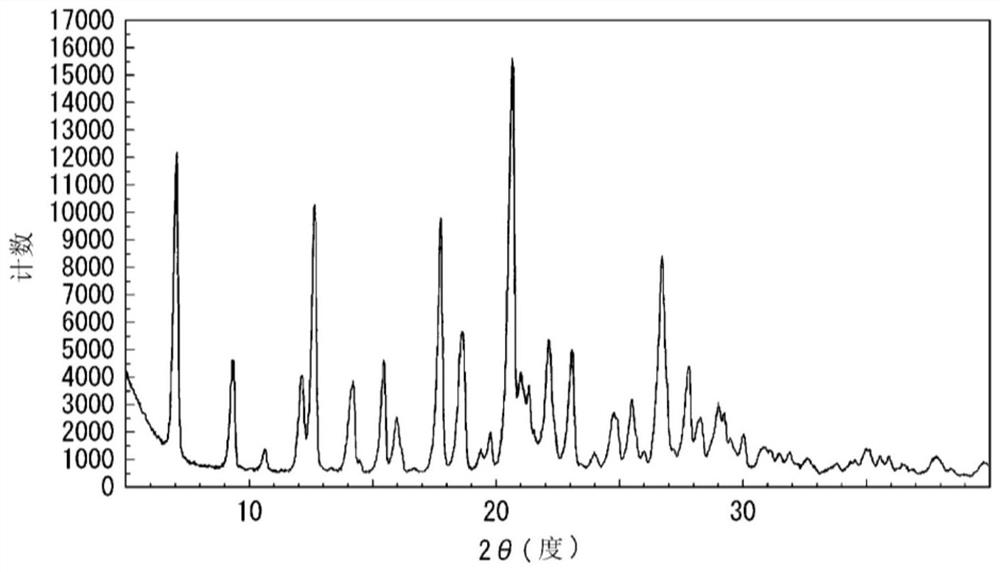
reference example 2
[0279]
[0280] Compound 14 was dissolved in tetrahydrofuran to prepare 2.5 mg / mL. Each polymer shown in Table 4B was dissolved in a mixed solvent (methanol / dichloromethane=3 / 4) to prepare about 45 mg / mL. The compound 14 solution was added to the above polymer solution while stirring so that the weight ratio of the compound 14 to the polymer became 1:25. In addition, a sample in which no polymer was added was also prepared. Immediately, the mixture was transferred to an eggplant-shaped flask, and the organic solvent was distilled off with a rotary evaporator (N-1100, Tokyo Rikagaku Co., Ltd.). The eggplant-shaped flask was moved to a desiccator, and dried under reduced pressure using a vacuum pump for about 16 hours to obtain a solid dispersion of compound 14. After the solid dispersion was dried, it was pulverized with an agate mortar or a portable high-speed pulverizer (LM-PLUS, Osaka Chemical Co., Ltd.), and then sieved (mesh: 150 μm).
[0281] [Form 4B]
[0282] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com