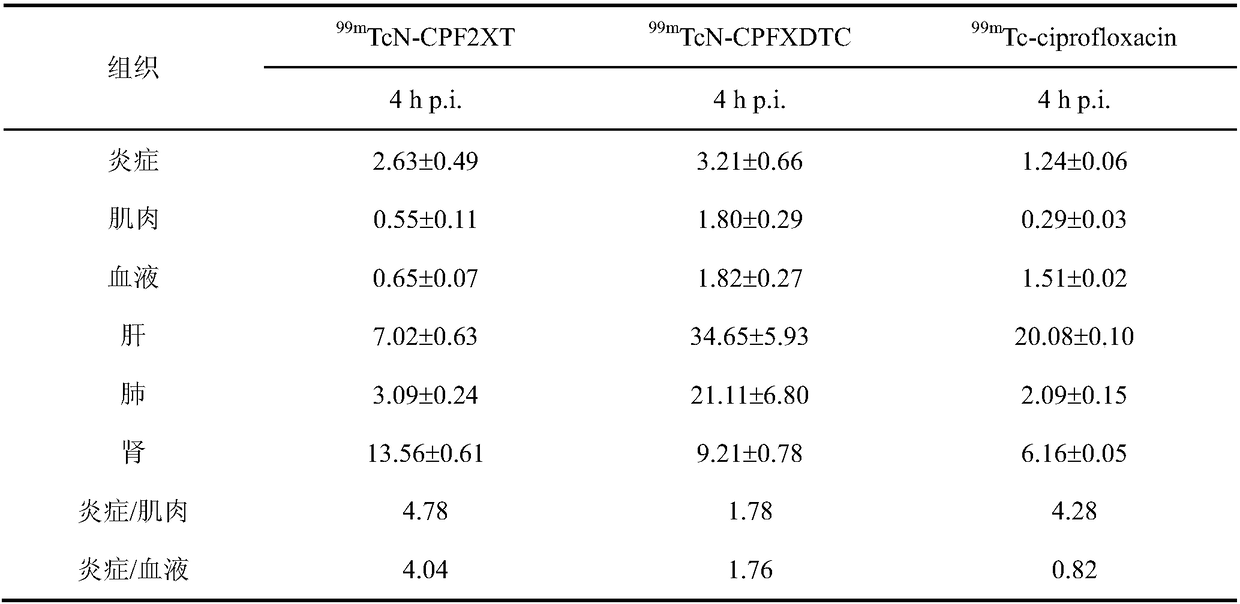
Tc-99m marked ciprofloxacin xanthate complex, as well as preparation method and application thereof
A technology of ciprofloxacin and xanthate, applied in the fields of radiopharmaceutical chemistry and clinical nuclear medicine, can solve the problems of low specificity, false positives and low labeling rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
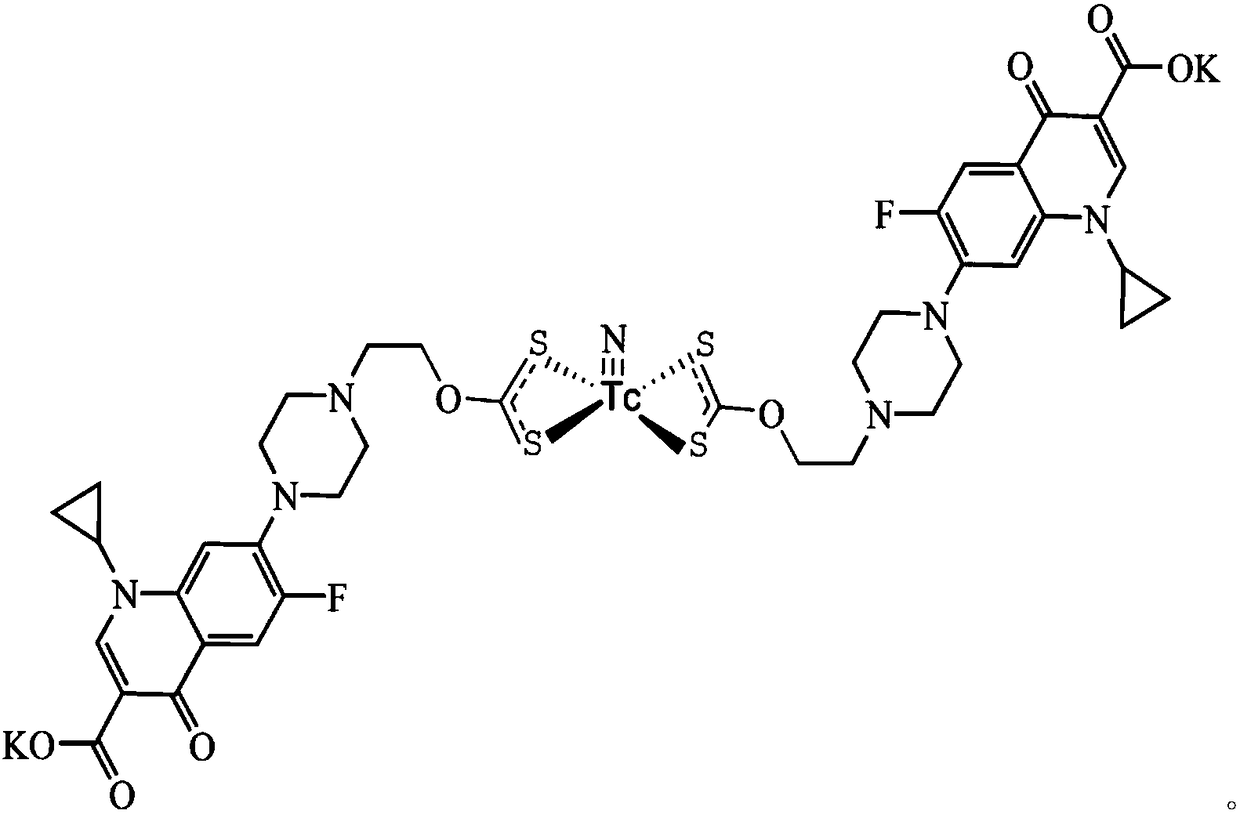
[0043] Describe the present invention in detail below by embodiment: a kind of 99m TcN-CPF2XT complex, its structural formula is as follows:
[0044]
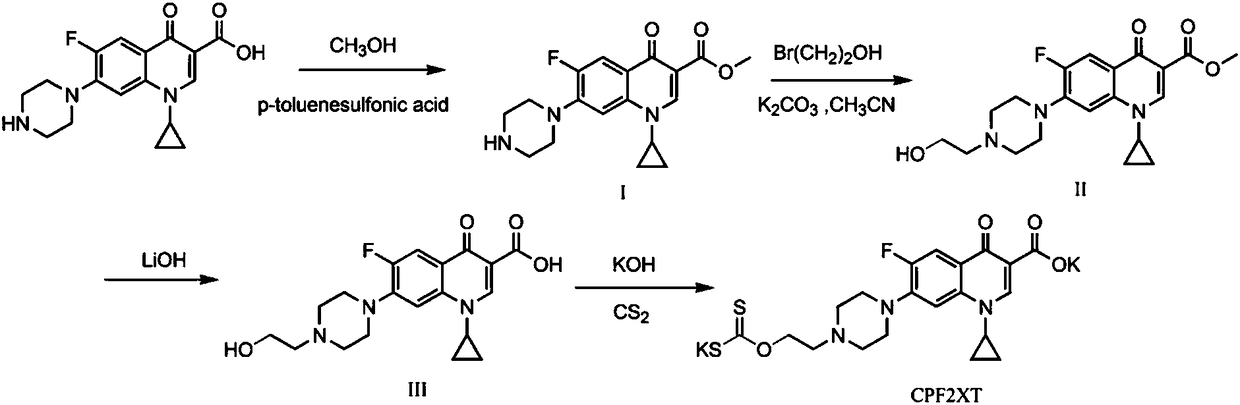
[0045] a. Synthesis of Ligand CPF2XT
[0046] Weigh 2.00g (6.00mmol) of ciprofloxacin, dissolve it in 100mL of anhydrous methanol, slowly add 1.72g (8.90mmol) p-toluenesulfonic acid, stir until the mixture is uniform and fully dissolved. Heated in an oil bath at 80°C, stirred and refluxed for 12 hours, and cooled to room temperature. Slowly add NaHCO 3 solution, with CH 2 Cl 2 Extraction (100mL×3), the organic phases were combined and dried over anhydrous magnesium sulfate, filtered, and rotary evaporated to obtain a white solid, compound I;
[0047] Weigh compound I1.00g (2.88mmol), K 2 CO 3 1.20g (8.64mmol) and bromoethanol 0.72g (5.76mmol), add 50mL of acetonitrile as solvent, heat in an oil bath at 80°C, stir and reflux for 12h; add appropriate amount of deionized water to the reaction system to dissolve, and use ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com