18F marked methylguanidine substituted benzene analogue and application thereof
A technology of guanidinium methyl and analogs, applied in the direction of isotope introduction into organic compounds, organic chemistry, organic chemistry methods, etc., can solve problems affecting cardiac visualization, poor biological performance, low spatial and temporal resolution, etc., and achieve good heart Effects of initial uptake and retention, good target to non-target ratio, high radiochemical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
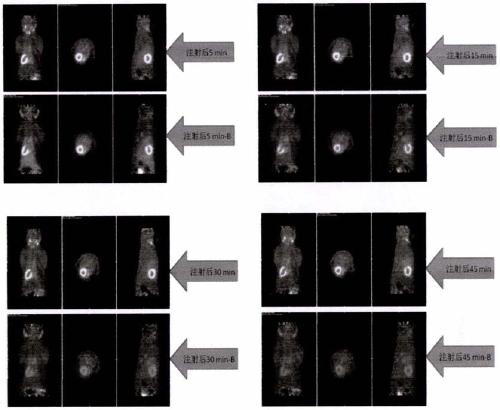
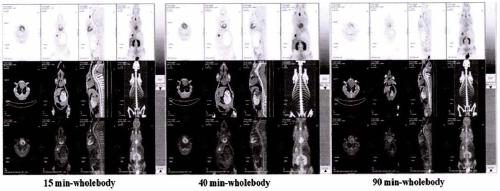
Examples
Embodiment Construction
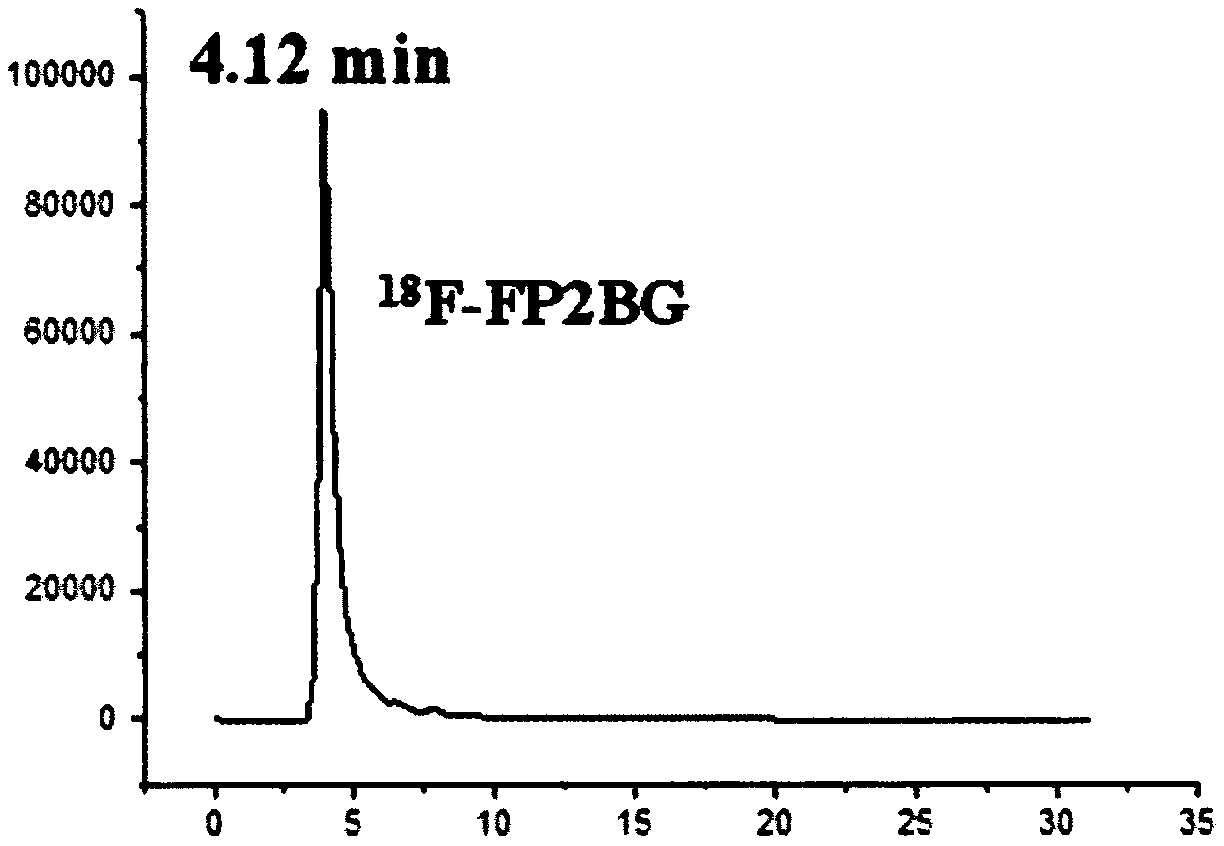
[0039] Below to 18 Taking F-FP2BG as an example, the present invention is described in detail through examples, but the present invention is not limited to these examples.
[0040] 1. Preparation of compounds
[0041] Materials: Compound 1 and Compound 3 in the following synthetic route were prepared by ourselves. Potassium carbonate, cesium carbonate, anhydrous acetonitrile, K 222 , anhydrous DMSO were purchased from Sigma / Aldrich. 18 f - From Atomic Hi-Tech Co., Ltd.
[0042] HPLC instruments and methods:
[0043] (1) Instrument:
[0044] Waters 1525 binary high-pressure liquid chromatography system, 2998 full-wavelength UV detector, RaytestGabiStar radioactivity detector, chromatographic column: Kromasil C18 5μm 10*250 mm
[0045] (2) method:
[0046] isocratic elution
[0047] Mobile phase: a mixture of 30mM ammonium acetate buffer (pH=6.8) and acetonitrile (volume ratio 1:3)
[0048] Flow rate: 5mL / min
[0049] 18 Preparation of F-FP2BG:
[0050]
[0051] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com