Multifunctional imaging probe and preparation method and application thereof
A multifunctional and functional technology, applied in the preparation method of peptides, chemical instruments and methods, X-ray contrast agent preparation, etc., can solve the problems of insufficient development of optical probes and achieve good tumor-targeted fluorescence imaging functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
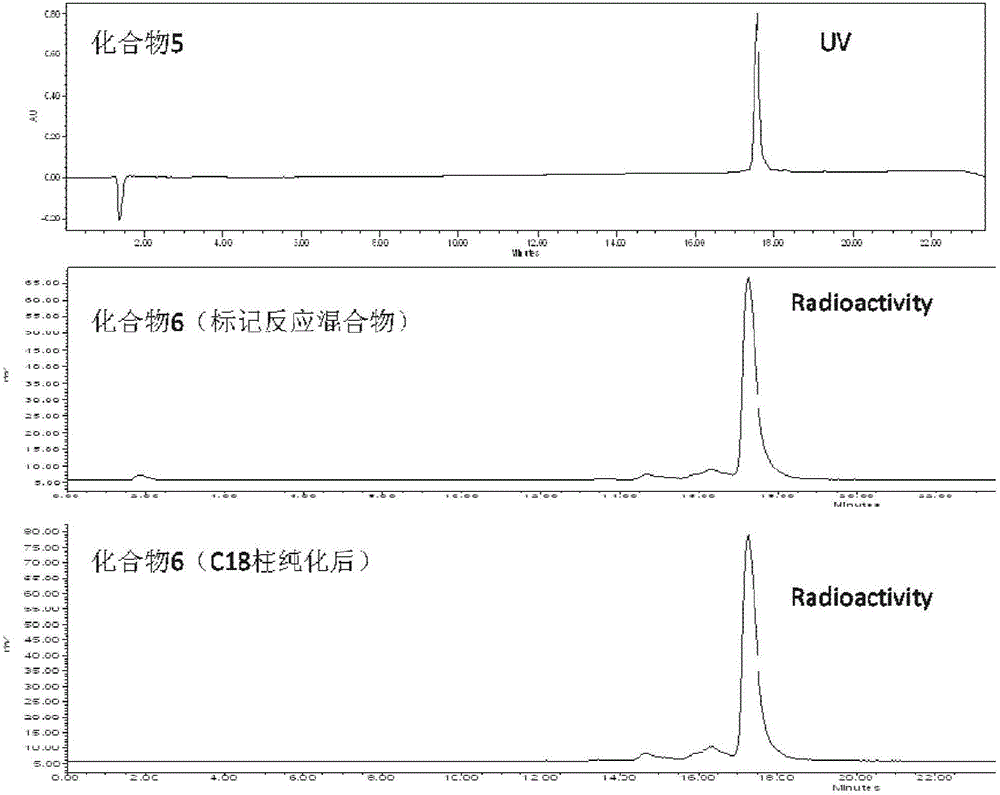
[0069] Prepare a polypeptide compound (compound 5) with tumor-targeted optical imaging function, and its synthetic route is as follows:
[0070]
[0071] Concrete preparation process comprises the following steps:
[0072] 1) Synthesis of Compound 2:
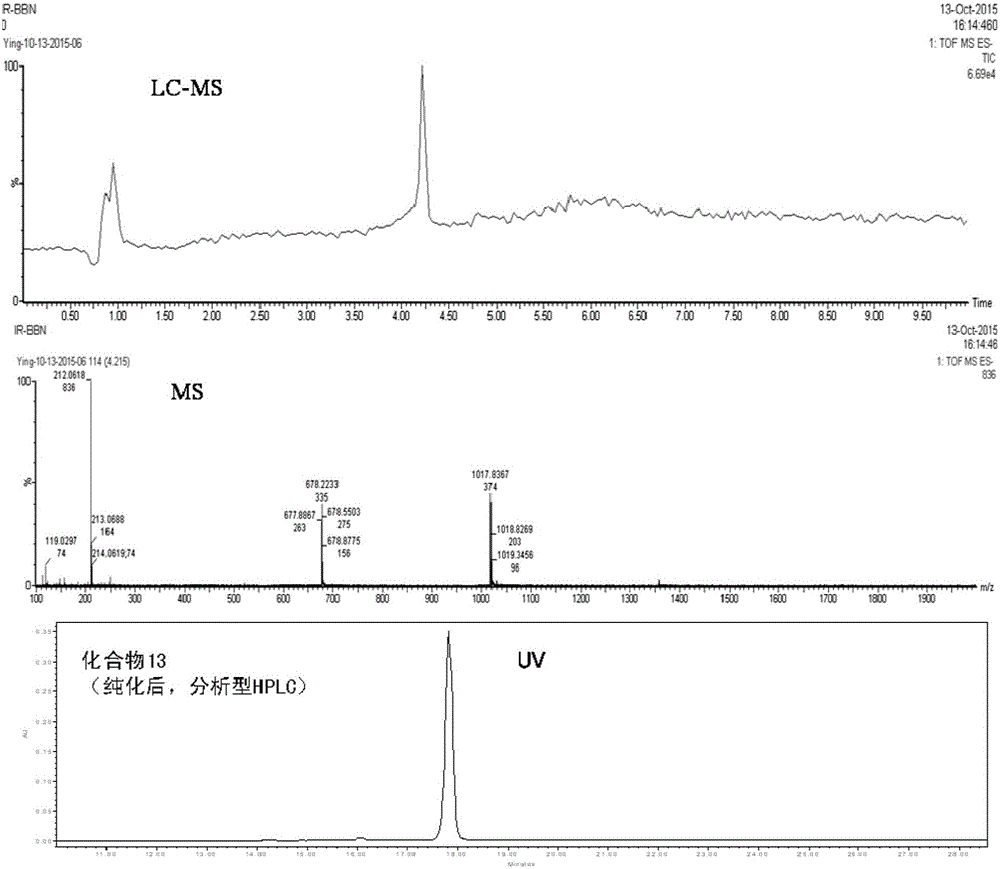
[0073] Add 9.5mg of Fmoc-Lys(Boc)-OH (lysine protected by Fmoc and Boc), 25μL of diisopropylethylamine (DIPEA) and 5μL of diethylphosphoric acid chloride (DECP) mixture into 27.5mg BBN (commercially available tumor-targeting polypeptide dissolved in 2.6 mL of dimethylformamide (DMF)) in a 20 mL glass vial. After mixing and dissolving, it was stirred at room temperature for 2 h, and then analyzed by liquid chromatography and mass spectrometry (LC-MS), the results showed that the target compound 1 was generated. Immediately after adding 0.6 mL of piperidine to the compound 1 solution and continuing to stir at room temperature for 1 h, the Fmoc protecting group was removed to obtain the target product (compound 2). After sepa...
Embodiment 2
[0088] 1. Radioactivity 64 Cu and 68 Preparation of freeze-dried kits for Ga labeling (take the preparation of 100 as an example)
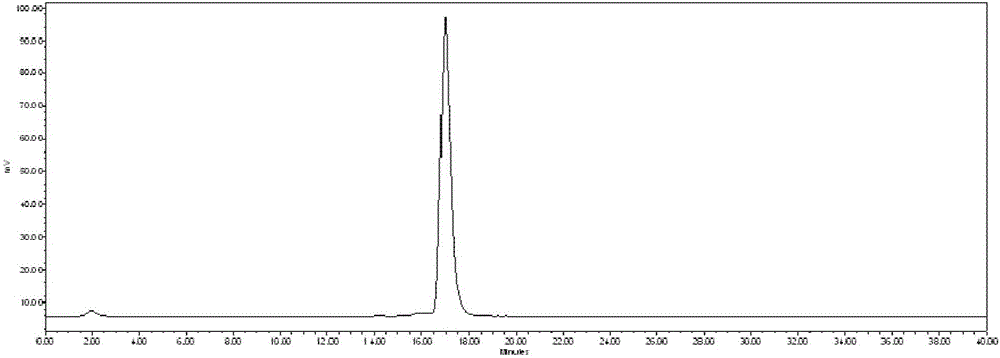
[0089] Weigh 4 mg of compound 5 (or 9) prepared in Example 1 and dissolve it in 10 mL of 0.5 mol / L acetic acid-sodium acetate buffer solution (pH 4), and distribute it in 100 cryopreservation tubes after sterile filtration, and then Place it in a freeze dryer for freeze-drying for 24 hours, and seal it with a stopper to obtain the freeze-dried medicine box I. According to the molding conditions of the freeze-dried powder of the kit, excipients, such as mannitol, ascorbic acid, etc., can be added to the kit, and the dosage of compound 5 (or 9) and excipients can be adjusted to achieve the best molding of the kit.
[0090] 2. Radioactivity 18 Preparation of freeze-dried kits for F labeling (take the preparation of 100 as an example)
[0091] Weigh 4mg of compound 5 (or 9) prepared in Example 1 and dissolve it in 10mL of 0.5mol / L tartaric acid-po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com