Polypeptide-protein-drug-carried solid particulate matter and double-enteric solid preparation containing same, and preparing methods and application of polypeptide-protein-drug-carried solid particulate matter and double-enteric solid preparation
A technology of solid particles and protease inhibitors, which is applied in the direction of peptide/protein components, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of increasing costs, complicated preparation processes, and limited production scale and other problems, to achieve the effect of easy realization, simple preparation process and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
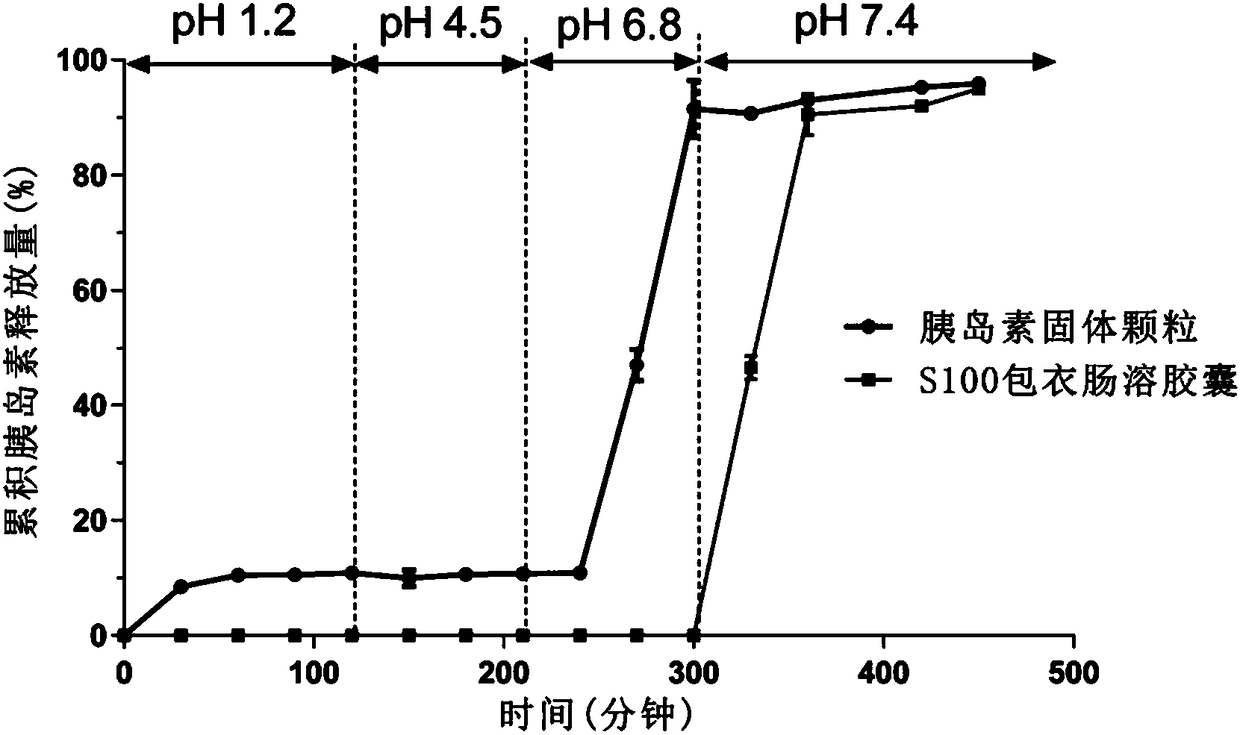
[0060] Preparation Example 1 Preparation of Eudragit S100-coated insulin granules
[0061] Dissolve 100mg of SNAC in 1mL of 0.01M NaOH, and dissolve 10mg of insulin (Ins) in 1mL of 0.01M NaOH aqueous solution and add to the SNAC solution to form a homogeneous solution (A); add 500mg of Eudragit S100 to 48mL of tert-butanol medium (containing 5mL water), heated appropriately to make it fully dissolve to form a homogeneous solution (B); slowly add A to B, vortex mix at 500r / min to form a homogeneous solution, and use spray freeze-drying technology to prepare Eudragit S100 package of insulin granules.
preparation Embodiment 2
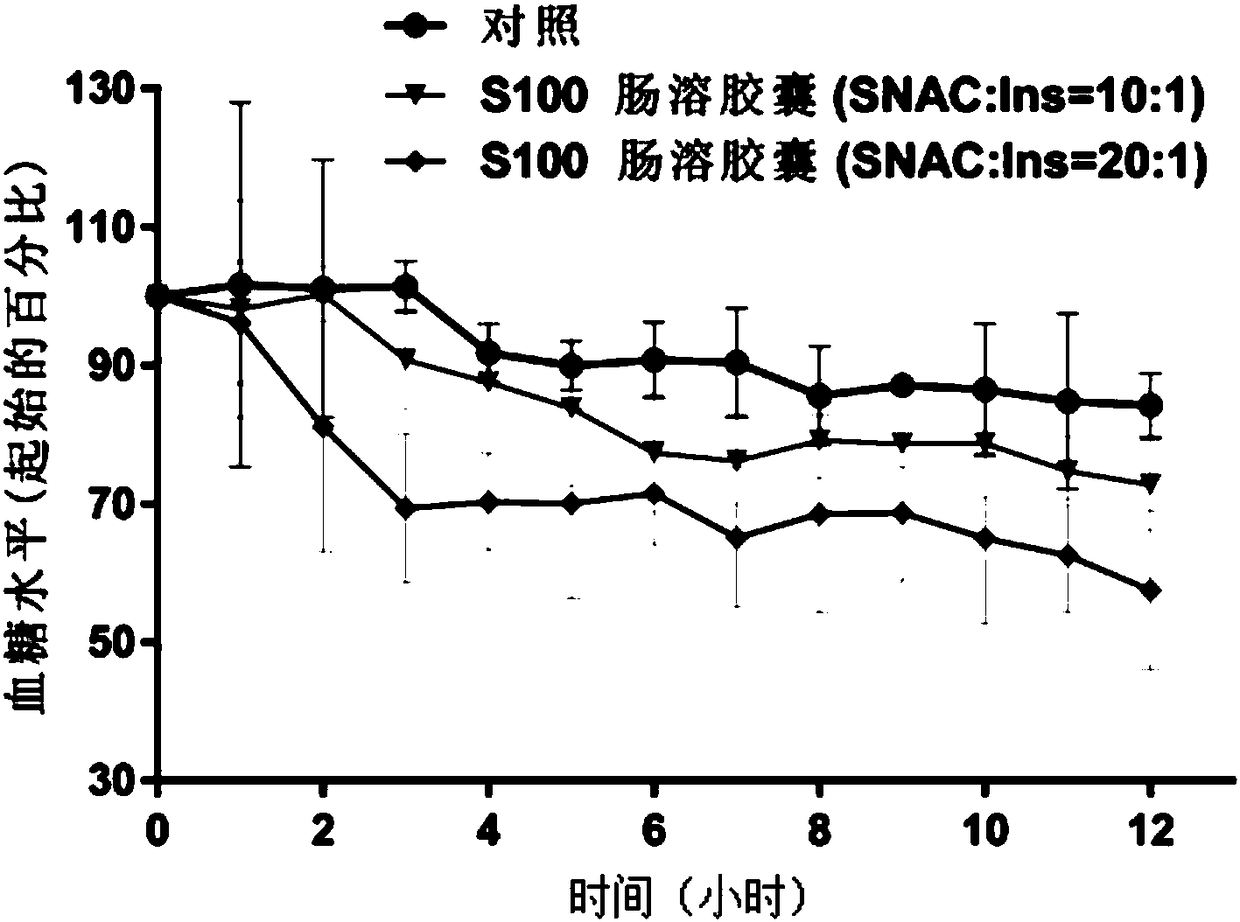
[0062] Preparation Example 2 Preparation of Eudragit S100-coated insulin granules containing protease inhibitors
[0063] Dissolve 100mg of SNAC in 1mL of 0.01M NaOH, and dissolve 10mg of Ins and 10mg of aprotinin in 1mL of 0.01M NaOH aqueous solution and add them to the SNAC solution to form a homogeneous solution (A); add 500mg of Eudragit S100 to 23mL of tert In butanol (containing 2mL water), heat appropriately to make it fully dissolve to form a homogeneous solution (B); slowly add A to B, vortex mix at 500r / min to form a homogeneous solution, and use spray freeze-drying technology to prepare Eudragit Insulin granules coated with S100.
preparation Embodiment 3
[0064] Preparation Example 3 Preparation of Eudragit S100-coated insulin granules containing protease inhibitors
[0065] Dissolve 200mg of SNAC in 1mL of 0.01M NaOH, and dissolve 10mg of Ins and 10mg of aprotinin in 1mL of 0.01M NaOH aqueous solution and add them to the SNAC solution to form a homogeneous solution (A); add 400mg of Eudragit S100 to 23mL of tert In butanol (containing 2mL water), heat appropriately to make it fully dissolve to form a homogeneous solution (B); slowly add A to B, vortex mix at 500r / min to form a homogeneous solution, and use spray freeze-drying technology to prepare Eudragit Insulin granules coated with S100.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com