A kind of phenoxyquinoline and its synthetic method
A technology of phenoxyquinoline and toluene, which is applied in the synthesis and application fields of fine chemical products, can solve the problems of unfavorable large-scale production of enterprises, many operation steps, and low total yield, and achieve low equipment requirements, few operation steps, Good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
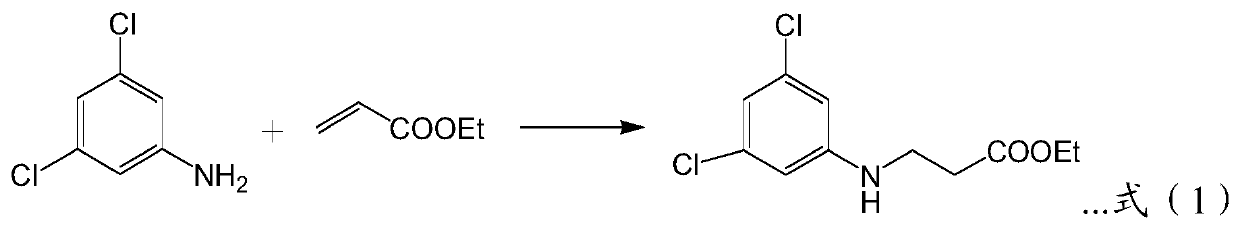
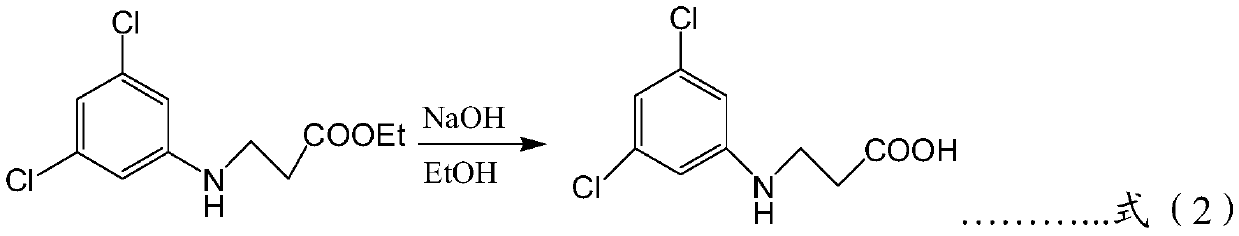
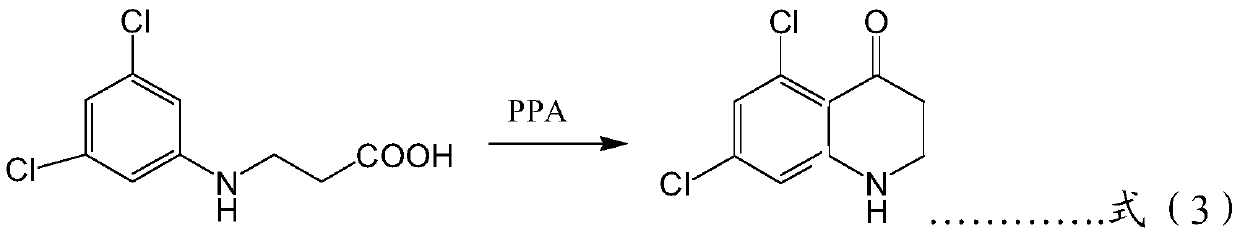
[0062] A preparation method for phenoxyquin, comprising the following preparation steps:
[0063] Step 1, add 60g of water, 120g of ethyl acrylate, 12g of zinc chloride, 5g of glacial acetic acid and 162g of 3,5-dichloroaniline into a 500mL three-neck flask, mix well, add 3,5-dichloroaniline and chloride After zinc, there is a white precipitate, heated to 99°C for 2.7 hours under reflux, stirred at a speed of 95 rpm during the reflux reaction until the white precipitate disappeared, then refluxed for 1.5 hours, then evaporated at 99°C for 1.8 hours to remove excess ethyl acrylate, cooled to room temperature, and suction-filtered for 10 minutes at a pressure of -0.087MPa and a temperature of 22.5°C to obtain a brownish-yellow solid, and then use 300g of 95% ethanol to recrystallize the brownish-yellow solid, specifically Method: first raise the temperature to 78°C, stir at this temperature at 95 rpm for 30 minutes, then lower the temperature to below 50°C to crystallize for 10 ...
Embodiment 2
[0078] A preparation method for phenoxyquin, comprising the following preparation steps:
[0079] Step 1, add 60g of water, 100g of ethyl acrylate, 10g of zinc chloride, 7g of glacial acetic acid and 164g of 3,5-dichloroaniline into a 500mL three-neck flask, mix well, add 3,5-dichloroaniline and chloride After zinc, there is a white precipitate. Heat it to 98°C for 3 hours and reflux for 3 hours. During the reflux reaction, stir at a speed of 95 rpm until the white precipitate disappears, then reflux for 1.7 hours, and then evaporate at 100°C for 1.5 hours. Remove excess ethyl acrylate, cool to room temperature, and filter with suction for 8 minutes at a pressure of -0.085MPa and a temperature of 25°C to obtain a brownish-yellow solid, then use 280g of 95% ethanol to recrystallize the brownish-yellow solid , specifically: first raise the temperature to 75°C, stir at this temperature at 95 rpm for 25 minutes, and then lower the temperature to below 50°C to crystallize for 11 ho...
Embodiment 3
[0086] A preparation method for phenoxyquin, comprising the following preparation steps:
[0087] Step 1, add 60g of water, 140g of ethyl acrylate, 14g of zinc chloride, 3g of glacial acetic acid and 160g of 3,5-dichloroaniline into a 500mL three-neck flask, mix well, add 3,5-dichloroaniline and chloride After zinc, there is a white precipitate. Heat it to 98.5°C for 2.5 hours under reflux reaction. During the reflux reaction, stir at a speed of 95 rpm until the white precipitate disappears, then reflux for 1.3 hours, and then evaporate at 99.5°C for 2 hours. Remove excess ethyl acrylate, cool to room temperature, and filter with suction for 12 minutes at a pressure of -0.090 MPa and a temperature of 20°C to obtain a brownish-yellow solid, and then use 320 g of 95% ethanol to recrystallize the brownish-yellow solid , specifically: first raise the temperature to 81°C, stir at this temperature at 95 rpm for 35 minutes, and then lower the temperature to below 50°C to crystallize ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com