In-vitro amplification method of tumor infiltration lymphocyte (TIL)
A lymphocyte, in vitro expansion technology, applied in the biological field, can solve the problems of low expansion efficiency, limit the use of large amount of TIL reinfusion in tumor patients, and insufficient tumor lethality, so as to improve the lethality and promote the effect of in vitro proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1. Experimental materials
[0059] X-VIVO-15 medium was purchased from Lonza company; phosphate buffered saline (PBS), RPMI 1640 medium, CD3 monoclonal antibody, CD28 monoclonal antibody were purchased from Gibco company; human AB serum was provided by Huaian Blood Center; IL-2 It was purchased from Beijing Sihuan Biopharmaceutical Company; collagenase IV was purchased from Sigma Company; lymphocyte separation solution was purchased from Tianjin Meide Pacific Company.
[0060] The human hepatoma cell line HepG2 was cryopreserved by our company. After resuscitation, it was placed in RPMI 1640 medium containing 10% fetal bovine serum at 37°C and 5% CO. 2 Subculture in an incubator.
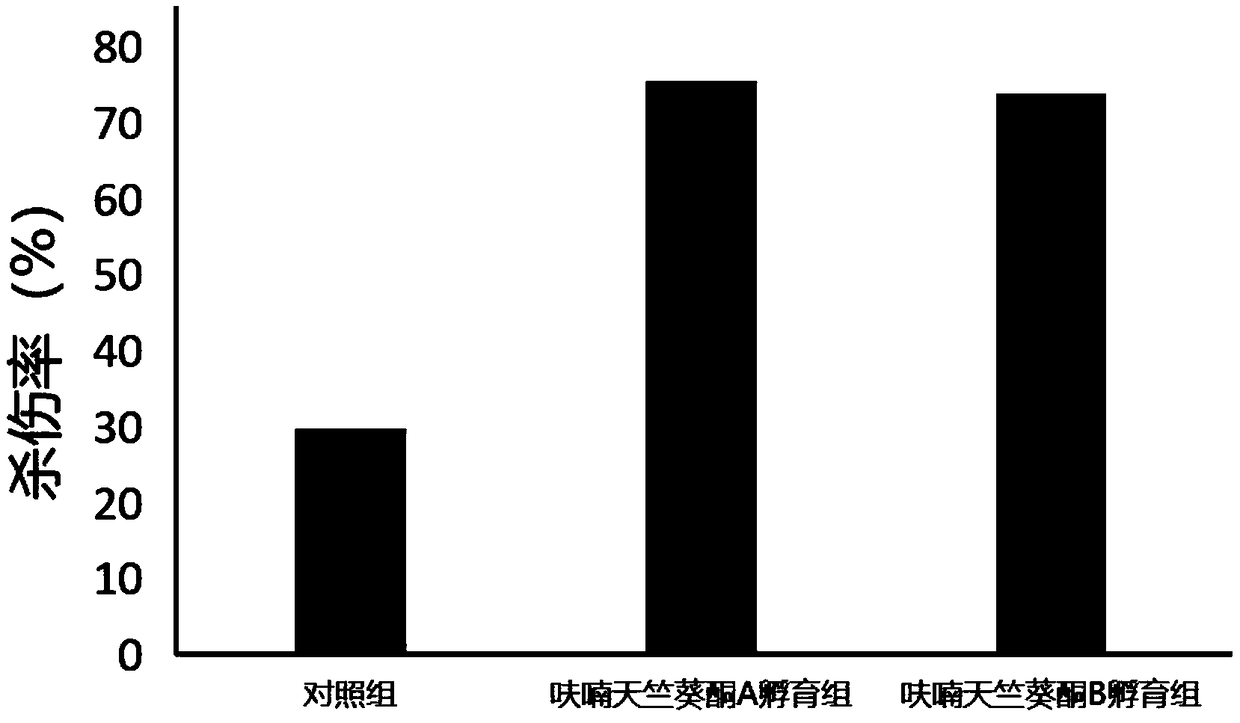
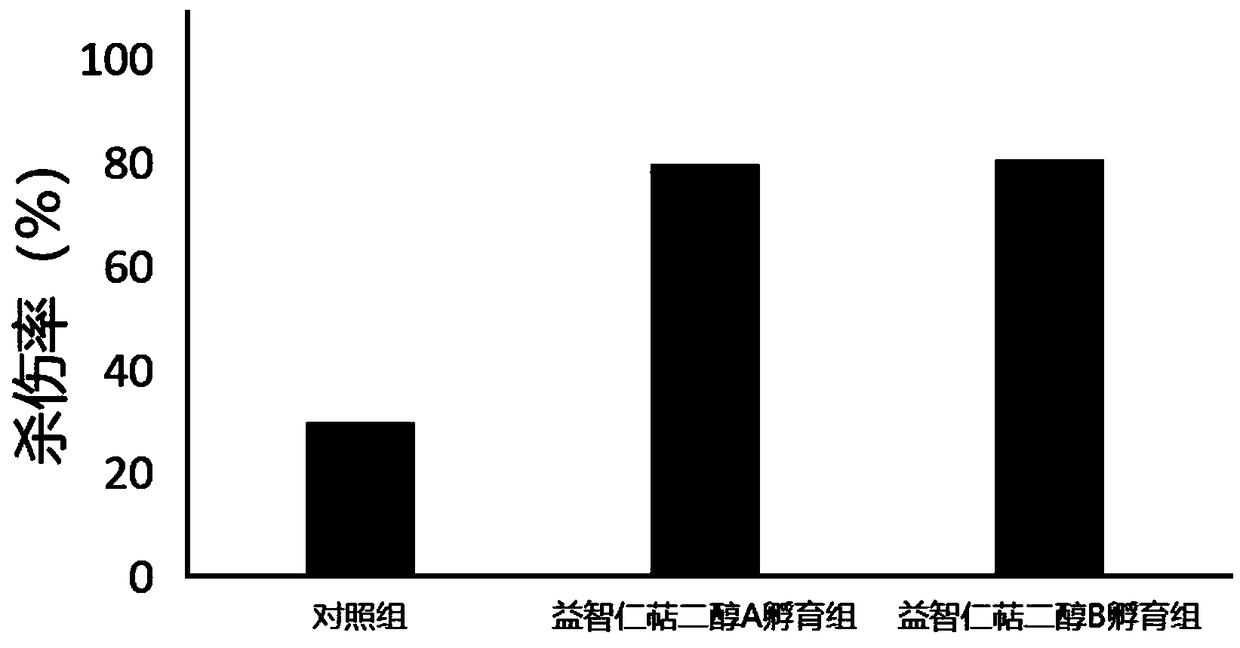
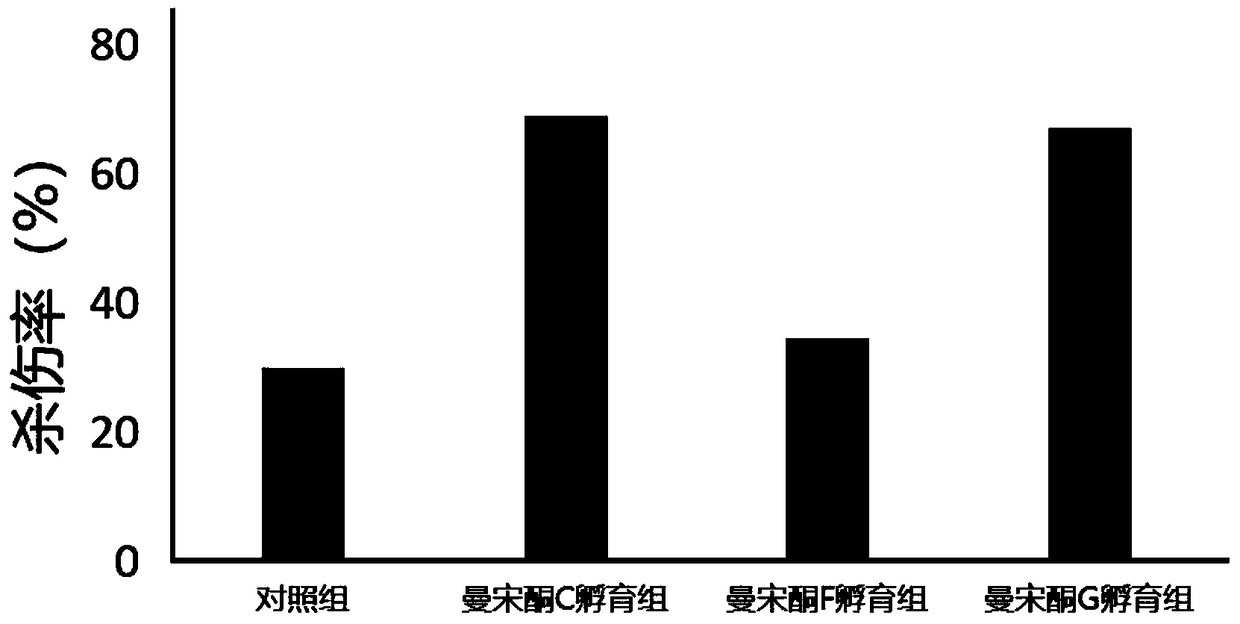
[0061] Furan geranone A (CAS number: 1143-45-9), furan geranone B (CAS number: 1143-46-0), nootropic terpene glycol A (CAS number: 363610-30-4), nootropic Arlendiol B (CAS No.: 363610-32-6), Manzonone C (CAS No.: 5574-34-5), Manzonone F (CAS No.: 5090-88-0), Manzonone G (CAS No.: 7715-96-0...
Embodiment 2
[0089] A kind of TIL cell culture medium, by adding 20 μg / mL of furan geranone A, furan geranone B, nozhirendiol A, nozhirendiol B, mansong in X-VIVO-15 medium Ketone C or Mansonone G. When used for culturing TIL cells, it is only necessary to add an appropriate amount of anti-CD3 monoclonal antibody and anti-CD28 monoclonal antibody, which is convenient to use.
[0090] In the method provided by the present invention, by adding a small amount of furan geranone A, furan geranone B, nootropic terpene diol A, nootropic terpene diol B, mansonone C or mansonone G in the culture medium, not only can It can significantly promote the proliferation of TIL cells in vitro, and can also significantly improve the lethality of TIL cells to tumor cells. Furanopelargonone A, furanopelargonone B, nootropic perolindiol A, nootropic perolindiol B, mansonone C or mansonone G can be used to prepare TIL cell culture medium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com