Application of a Curvularia lunata in C14α-Hydroxylation
A kind of Curvularia, hydroxylation technology, applied in the field of biotechnology and steroid drug transformation, can solve the problem of low pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
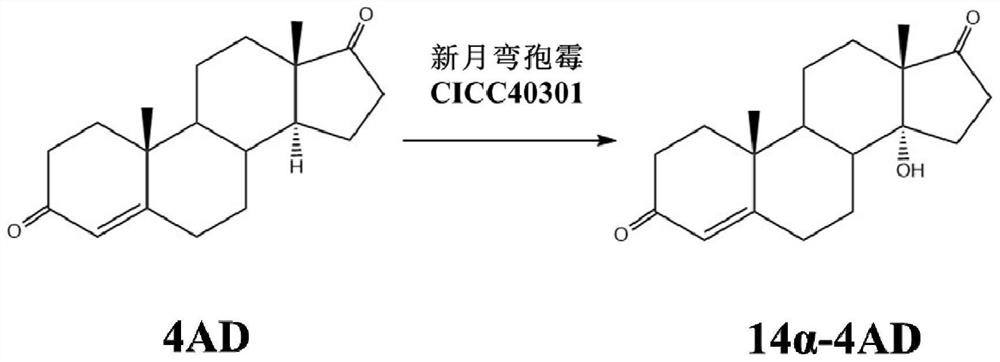
[0024] Embodiment 1 Curvularia crescenae CICC40301 transforms the method for 4AD
[0025] The reaction formula of Curvularia crescens transformation 4AD is as follows figure 1 shown.
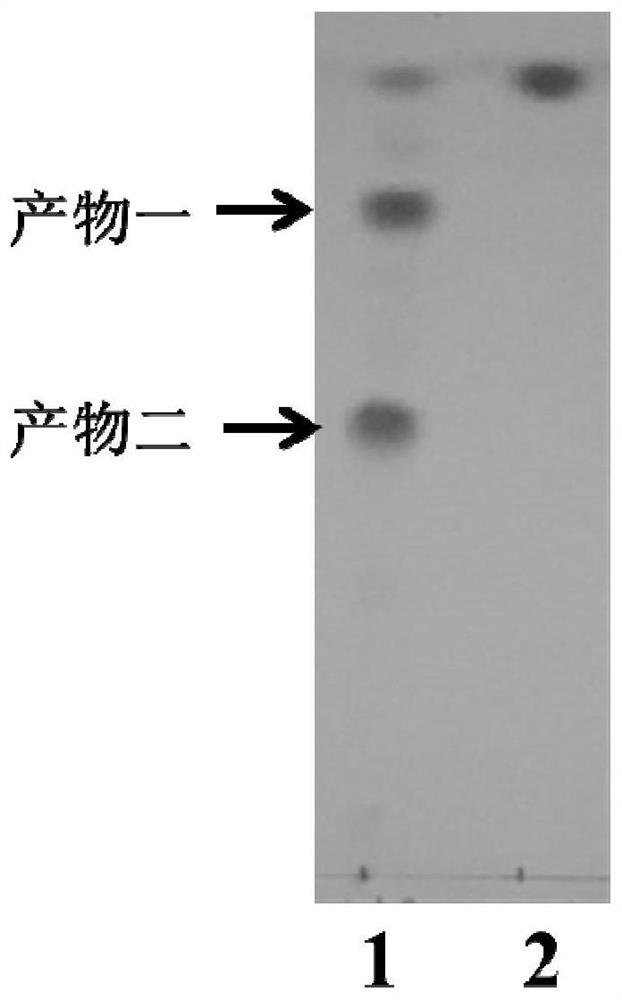
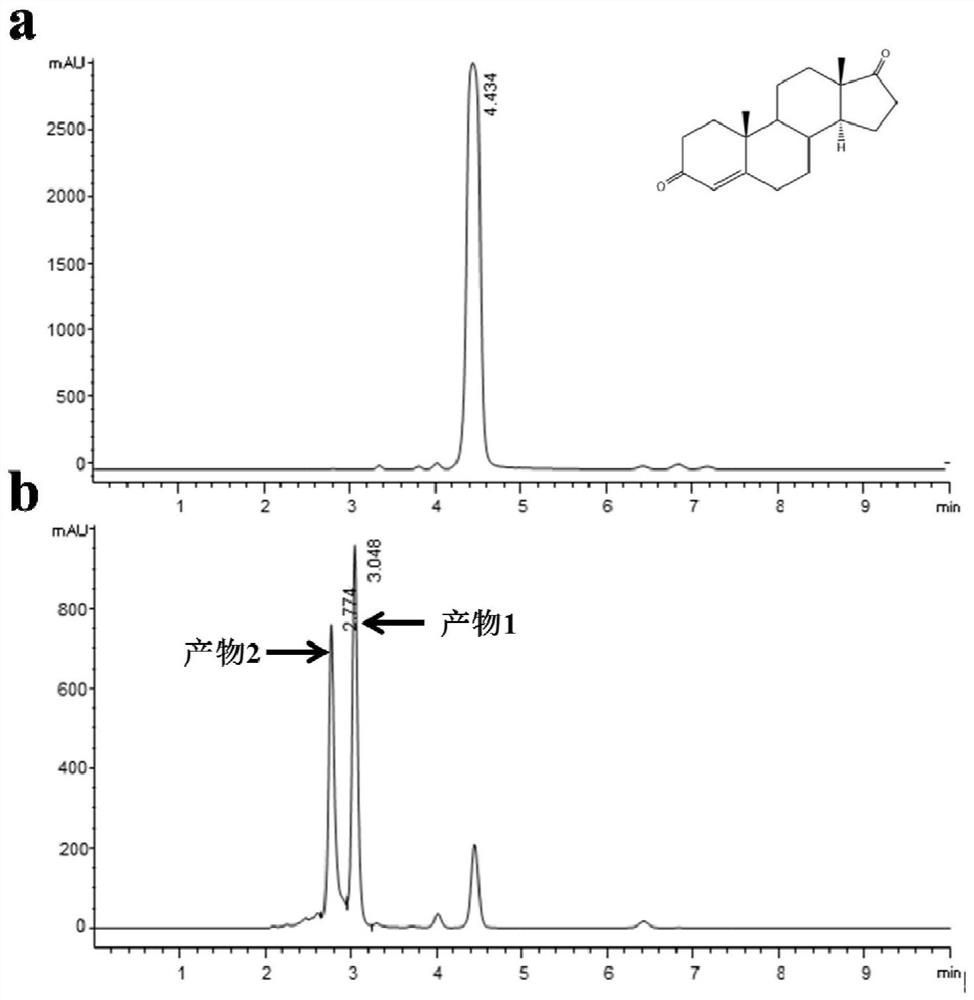
[0026] Take 200 μL of the Curvularia crescens strain seed solution and connect it to 50 mL of YPD liquid medium, and after cultivating at 28°C for 36 hours, add 0.2% androst-4-ene-3,17-dione substrate (4AD), and then After 48 hours of conversion, the yield of C14α-4AD was 48.3%.
Embodiment 2
[0027] Embodiment 2 Curvularia crescenae CICC40301 transforms the method for 4AD
[0028] Curvularia crescenae CICC40301 was inoculated in YPD liquid medium at an inoculum of 1% after seed cultivation, and after culturing at 25°C for 24 hours, 0.1% androst-4-ene-3,17-dione was added Object (4AD), then transform 24h;
[0029] After 24 hours of transformation, the yield of C14α-4AD reached 41.5%.
Embodiment 3
[0030] Embodiment 3 Curvularia crescenae CICC40301 transforms the method for 4AD
[0031] After Curvularia lunae CICC40301 was cultured by seeds, it was inoculated in YPD liquid medium at an inoculum size of 2%, and after culturing at 30°C for 36 hours, 0.3% of androst-4-ene-3,17-dione was added to it. Object (4AD), then transform 40h;
[0032] After 40 hours of conversion, the yield of C14α-4AD reached 45.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com