A kind of method for preparing difluoroacetate by gas-solid phase reaction
A technology of difluoroacetate and difluorochloroacetate alkyl ester is applied in the field of preparing difluoroacetate by gas-solid phase reaction, and can solve the problems of equipment corrosion, difficulty in obtaining difluoroacetonitrile, long process flow and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1, Pd / AC catalyst (sample A)
[0056] Grind the activated carbon and screen out 10-20 meshes as a carrier, weigh 100g of activated carbon, put it into 300ml of hydrochloric acid solution with a concentration of 10%, in a water bath with a temperature of 80°C, stir and reflux for 6 hours, take it out, and dissolve it with distilled water The acid-washed activated carbon was washed to neutrality, and then dried at 100°C for use.
[0057] Weigh 0.77g of PdCl 2 , dissolved in 2ml of concentrated hydrochloric acid, added 15g of distilled water to make PdCl 2 After fully dissolving, add 2g of citric acid, add 14.5g of the above acid-washed activated carbon to the above solution, impregnate for 24h, and freeze-dry and dehydrate at -20°C for 20h. Roasting in a nitrogen atmosphere for 4h, cooled for later use.
Embodiment 2
[0058] Embodiment 2, Pd-Ce / AC catalyst (sample B)
[0059] Weigh 0.93g of Ce(NO 3 ) 3 ·6H 2 O, dissolved in 15g of distilled water, after fully dissolving, add 15g of activated carbon treated in Example 1, impregnate for 24h, dry at 100°C, then roast at 400°C for 4h in a nitrogen atmosphere, cool and set aside.
[0060] Weigh 0.51g of PdCl 2 , dissolved in 2ml of concentrated hydrochloric acid, added 10g of distilled water to make PdCl 2 After fully dissolving, add 1.3g of citric acid, then add 10g of activated carbon containing additive Ce after the above-mentioned treatment, impregnate for 24h, and freeze-dry and dehydrate at -20°C for 20h. Calcined in nitrogen atmosphere for 4h, cooled for later use.
Embodiment 3
[0061] Embodiment 3, preparation of methyl difluoroacetate
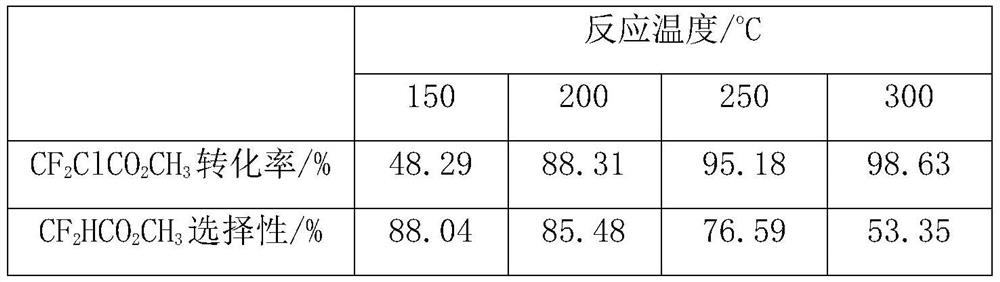
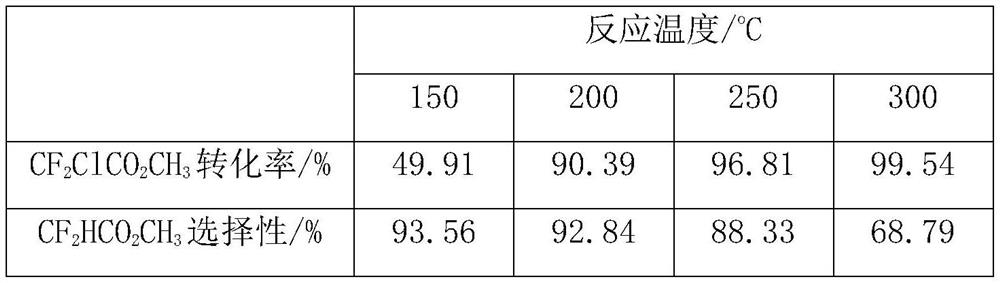
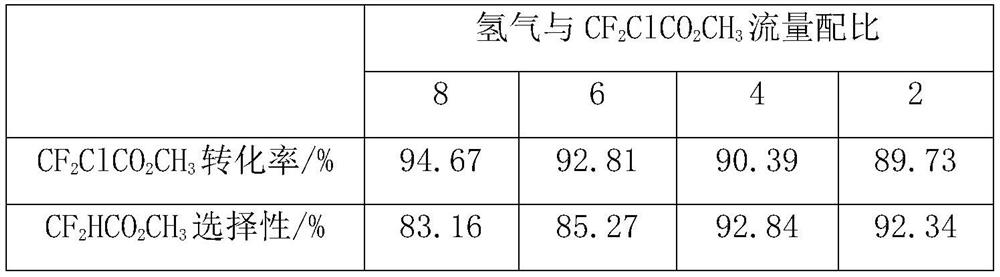
[0062] The sample A is used as a catalyst for preparing methyl difluoroacetate by catalytic hydrodechlorination of methyl difluorochloroacetate. The reaction was carried out in a fixed bed reactor made of stainless steel tube (inner diameter: 20mm, length: 600mm), filled with 10ml, about 5g of sample A catalyst, and the reaction temperature was set at 150°C, 200°C, 250°C and 300°C, the operating pressure is normal pressure, the raw material methyl difluorochloroacetate is vaporized by the vaporizer (150°C) and mixed with hydrogen, and the space velocity of the raw material gas is about 30h -1 , the flow ratio of reducing gas hydrogen and methyl difluorochloroacetate is about 4.
[0063] The reaction product is analyzed and determined by gas chromatography analysis method, and then the product is collected by low-temperature condensation, and the unreacted hydrogen is vented. The results of the reaction analysis are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com