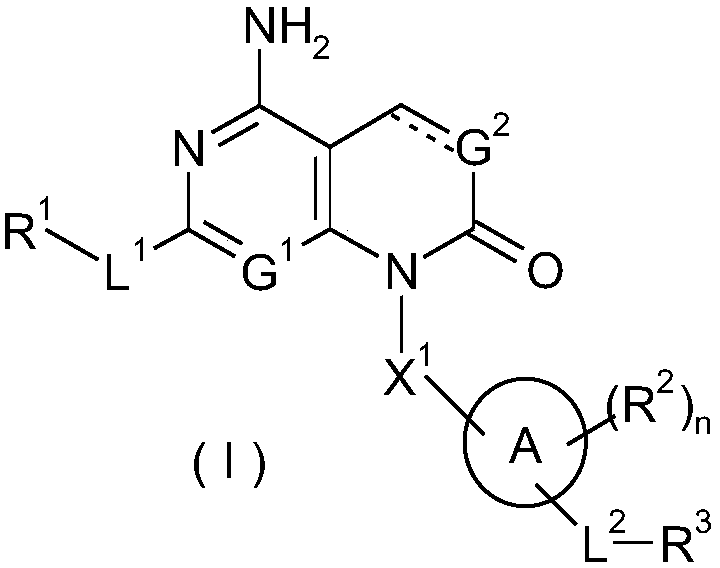
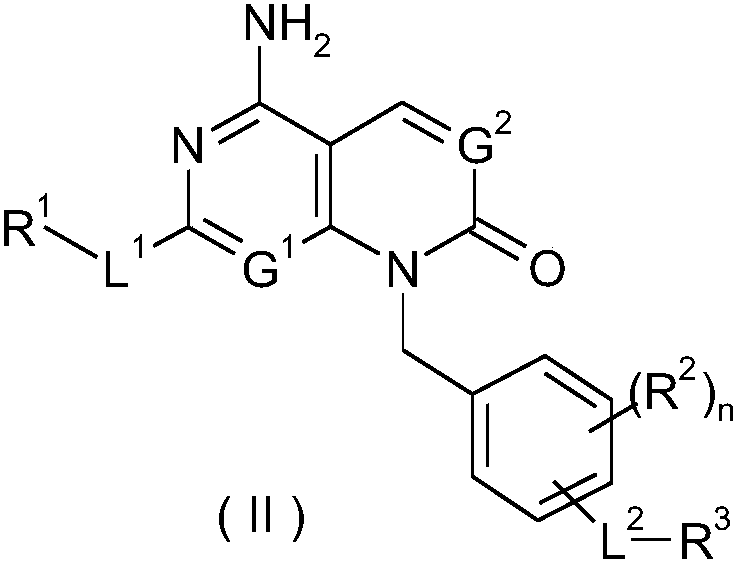
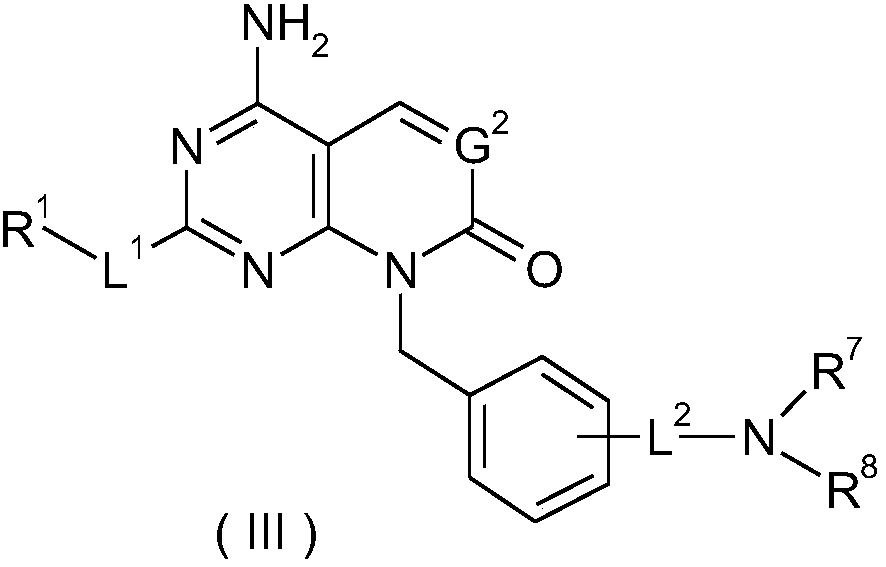
Condensed ring based ketone derivatives, preparation method thereof and application of derivatives in medicine
A technology of heterocyclyl and cycloalkyl, which can be used in the field of TLR7 agonists to solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0260] 5-Amino-7-butoxy-1-(4-(pyrrolidin-1-ylmethyl)benzyl)pyrimido[4,5-d]pyrimidin-2(1H)-one
[0261]
[0262]
[0263] first step
[0264] 2-Butoxy-4,6-dichloropyrimidine-5-carbaldehyde 1b
[0265] Dissolve phosphorus oxychloride (6.24g, 0.0407mol) in 75mL tetrahydrofuran, cool to 0°C, add N,N-dimethylformamide (2.98g, 0.0407mol), stir for 1 hour, then add 2- Butoxypyrimidine-4,6-diol 1a (5 g, 0.0271 mol, prepared by the method disclosed in the patent application "US20160075707") was stirred for 30 minutes, then raised to room temperature and stirred for 16 hours. The reaction solution was concentrated under reduced pressure, 50 mL of phosphorus oxychloride was added, cooled to 0°C, N,N-diisopropylethylamine (6.99 g, 0.0542 mol) was added, the temperature was raised to 60°C and the reaction was stirred for 2 hours. The reaction solution was cooled to room temperature, concentrated under reduced pressure, 50 mL of water was added to the resulting residue, extracted wi...
Embodiment 2
[0285] 4-Amino-2-butoxy-8-(4-(pyrrolidin-1-ylmethyl)benzyl)pyrido[2,3-d]pyrimidin-7(8H)-one
[0286]
[0287]
[0288] first step
[0289] 4-(bis(4-methoxybenzyl)amino)-2-butoxy-6-((4-(pyrrolidin-1-ylmethyl)benzyl)amino)pyrimidine-5-carbaldehyde 2b
[0290] Compound 1c (200mg, 0.425mmol) was dissolved in 10mL N,N-dimethylformamide, and (4-(pyrrolidin-1-ylmethyl)phenyl)methanamine 2a (121.5mg, 0.638mmol, Prepared by the known method "Journal of Medicinal Chemistry, 2012, 55(23), 10387-10404") and potassium carbonate (118mg, 0.85mmol), heated to 40°C and stirred for 2 hours. The reaction solution was cooled to room temperature, ethyl acetate was added, washed with saturated sodium chloride solution, the organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the obtained residue was purified with eluent system A by a CombiFlash rapid preparation apparatus , to obtain the title compound 2b (230 mg, yield: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com