Screening method and application of T cells with high anticancer activity
A screening method and anti-cancer activity technology, which is applied in the field of screening high anti-cancer active T cells, can solve the problems of few target cell subgroups, many useless cell subgroups, and low activity, and achieve strong and durable anti-tumor, tumor Long-lasting killing ability and strong re-stimulation amplification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
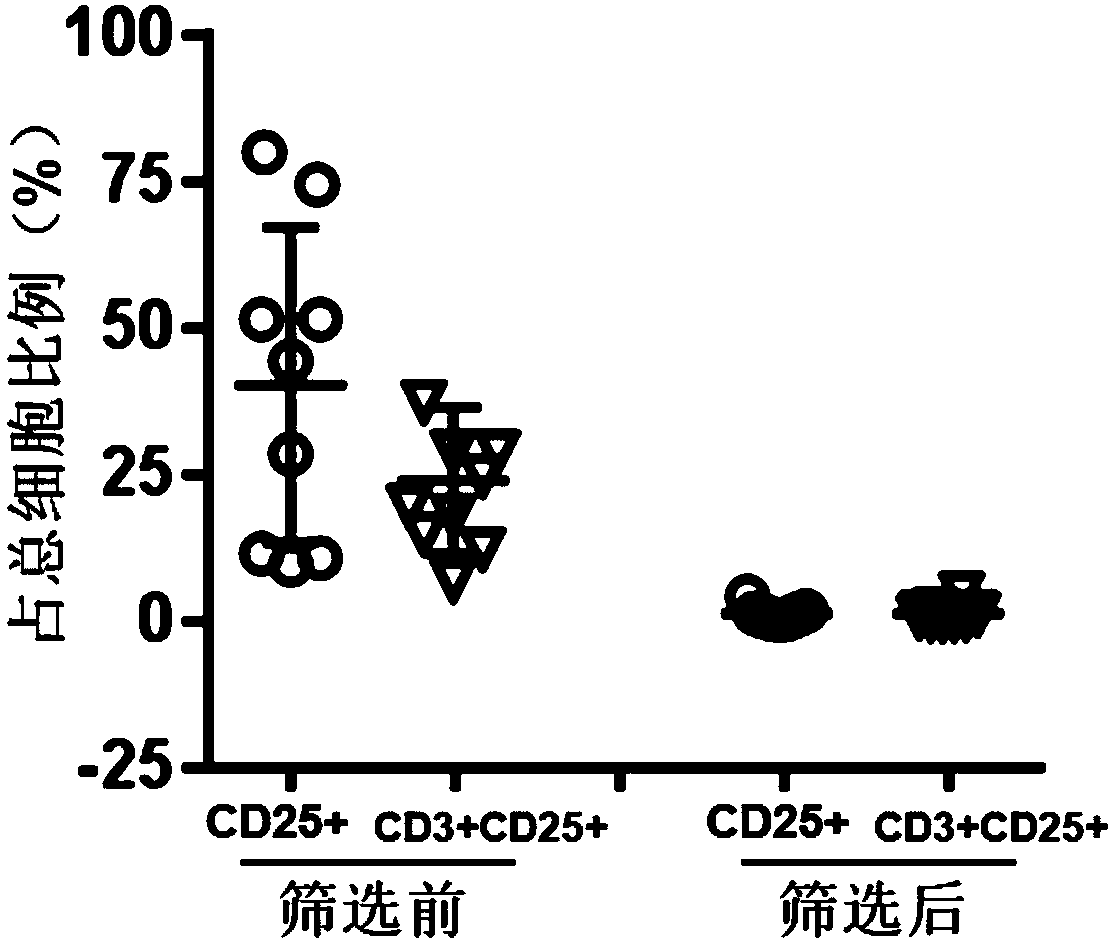
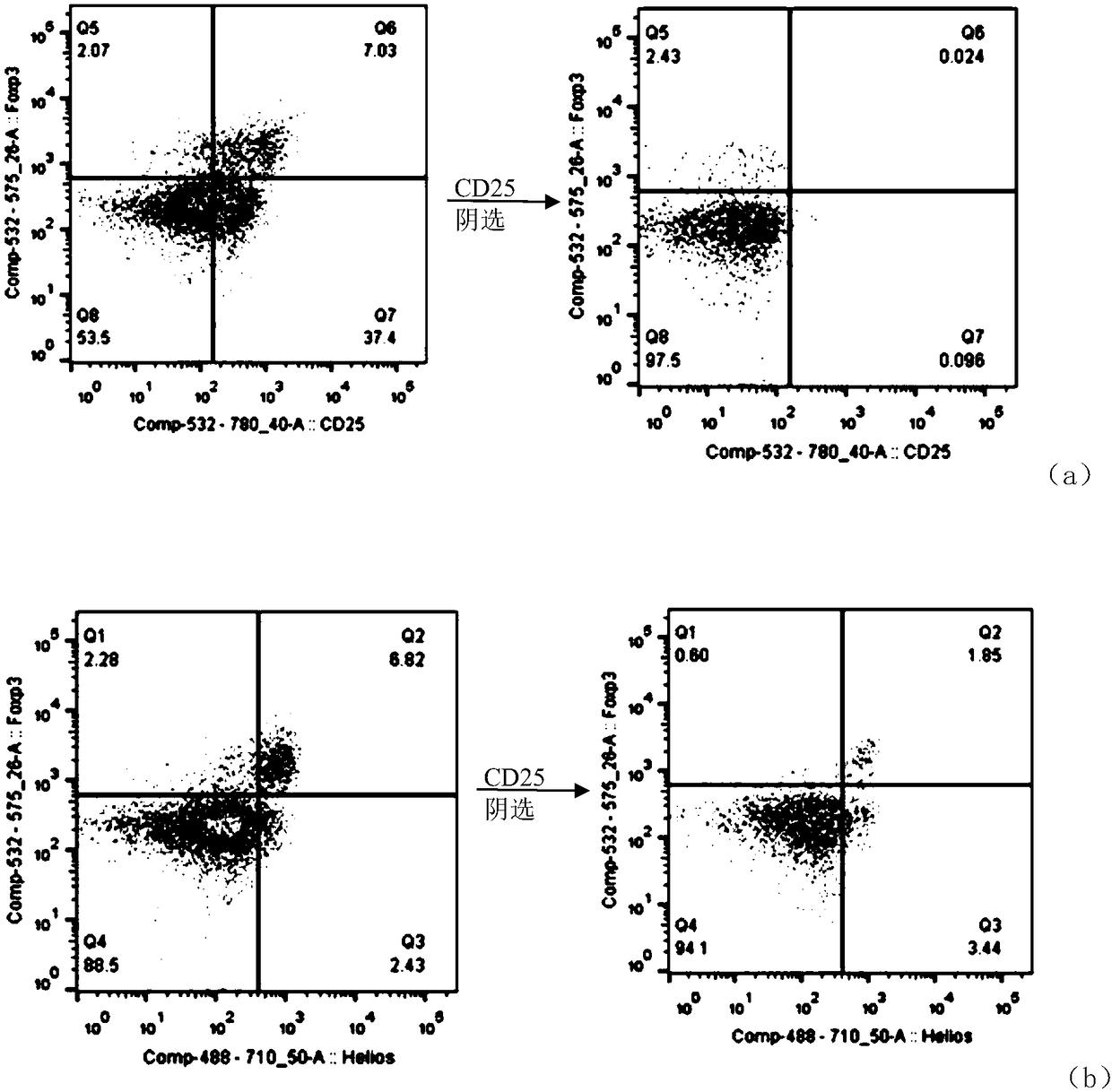
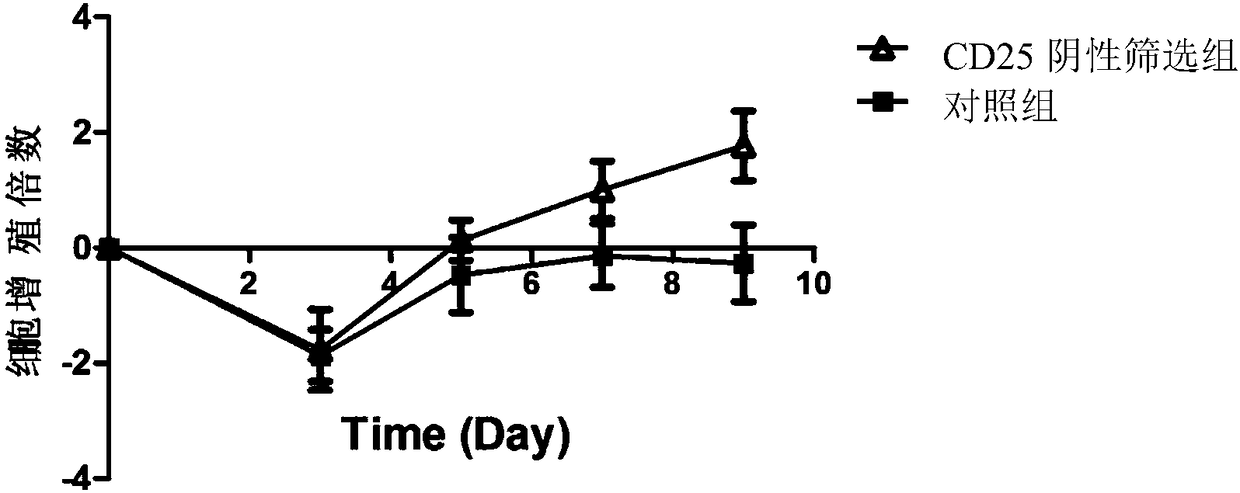
[0069] A screening method for T cells with high anticancer activity, comprising the following steps:
[0070] (1) Preparation of CD25 antibody-coated magnetic beads:
[0071] 1.1 Take 1mL of particle size of 4.5μm M-450Epoxy Loadable Magnetic Beads (hereinafter referred to as "magnetic beads") (40 million beads / mL), put them on a magnetic stand for 1 min, discard the supernatant, and take out the test tube from the magnetic stand;
[0072] 1.2 Add 1 mL of the first buffer solution (sodium phosphate buffer with pH=8.0) and repeat the washing twice;
[0073] 1.3 Resuspend the magnetic beads in the first buffer solution, add 200 μg of CD25 antibody, control the total volume to 1 mL, and mix well;
[0074] 1.4 Place the resulting mixed solution on a shaker or rotator at 20°C and gently shake, and incubate in the dark for 20 hours;
[0075] 1.5 Then add bovine serum albumin (BSA), control its concentration in the resulting dark incubation solution to be 0.02% (w / v), and then in...
Embodiment 2
[0097] A screening method for T cells with high anticancer activity, comprising the following steps:
[0098] (1) Preparation of CD25 antibody-coated magnetic beads:
[0099] 1.1 Take 1mL of particle size of 10μm M-450Epoxy Loadable Magnetic Beads (hereinafter referred to as "magnetic beads") (40 million beads / mL), put them on a magnetic stand for 1 min, discard the supernatant, and take out the test tube from the magnetic stand;
[0100] 1.2 Add 1 mL of the first buffer solution (sodium borate buffer with pH=8.4) and repeat the washing 3 times;
[0101] 1.3 Resuspend the magnetic beads in the first buffer solution, add 300 μg of CD25 antibody, control the total volume to 1 mL, and mix well;
[0102] 1.4 Place the resulting mixed solution on a shaker or rotator at 18°C and shake gently, and incubate in the dark for 24 hours;
[0103] 1.5 Then add bovine serum albumin (BSA), control its concentration in the resulting dark incubation solution to be 0.02% (w / v), and incubat...
Embodiment 3
[0121] A preparation method of CD25 antibody-coated magnetic beads, comprising the following steps:
[0122] 1.1 Take 1mL of particle size of 50-100μm M-450Epoxy Loadable Magnetic Beads (hereinafter referred to as "magnetic beads") (40 million beads / mL), put them on a magnetic stand for 1 min, discard the supernatant, and take out the test tube from the magnetic stand;
[0123] 1.2 Add 1 mL of the first buffer solution (Tris-hydrochloric acid buffer with pH=9.0) and repeat the washing 3 times;
[0124] 1.3 Resuspend the magnetic beads in the first buffer solution, add 150 μg of CD25 antibody, control the total volume to 1 mL, and mix well;
[0125] 1.4 Place the resulting mixed solution on a shaker or rotator at 35°C and shake gently, and incubate in the dark for 16 hours;
[0126] 1.5 Then add bovine serum albumin (BSA), control its concentration in the resulting dark incubation solution to be 0.02% (w / v), and then incubate at 35°C for 10min;
[0127] 1,6 After the incuba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com