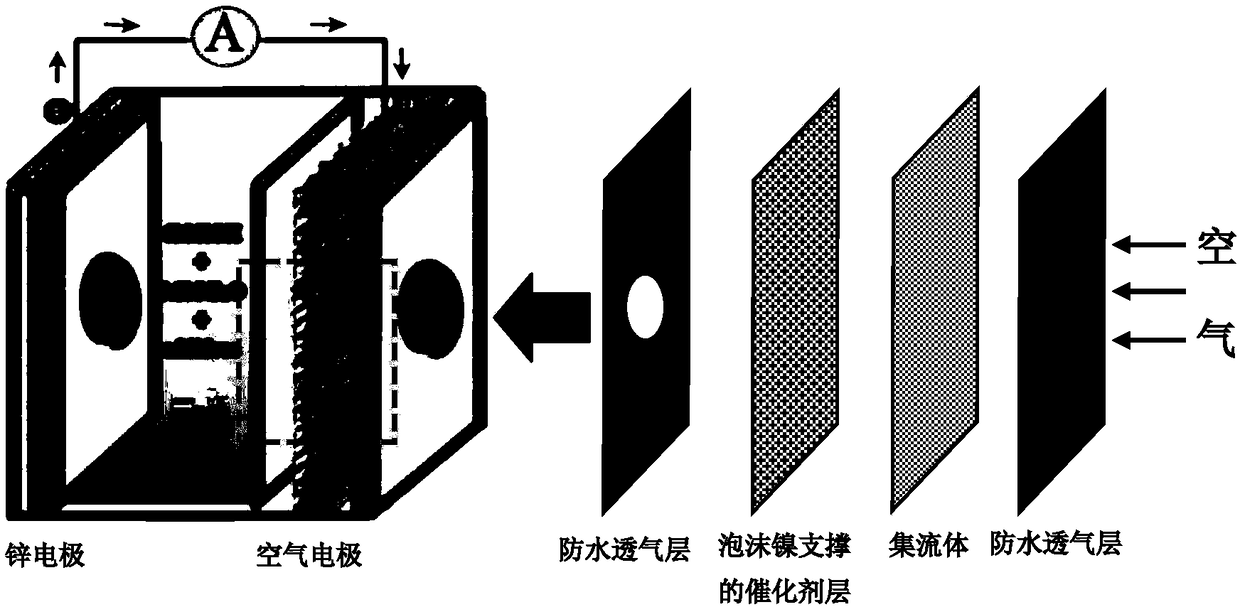
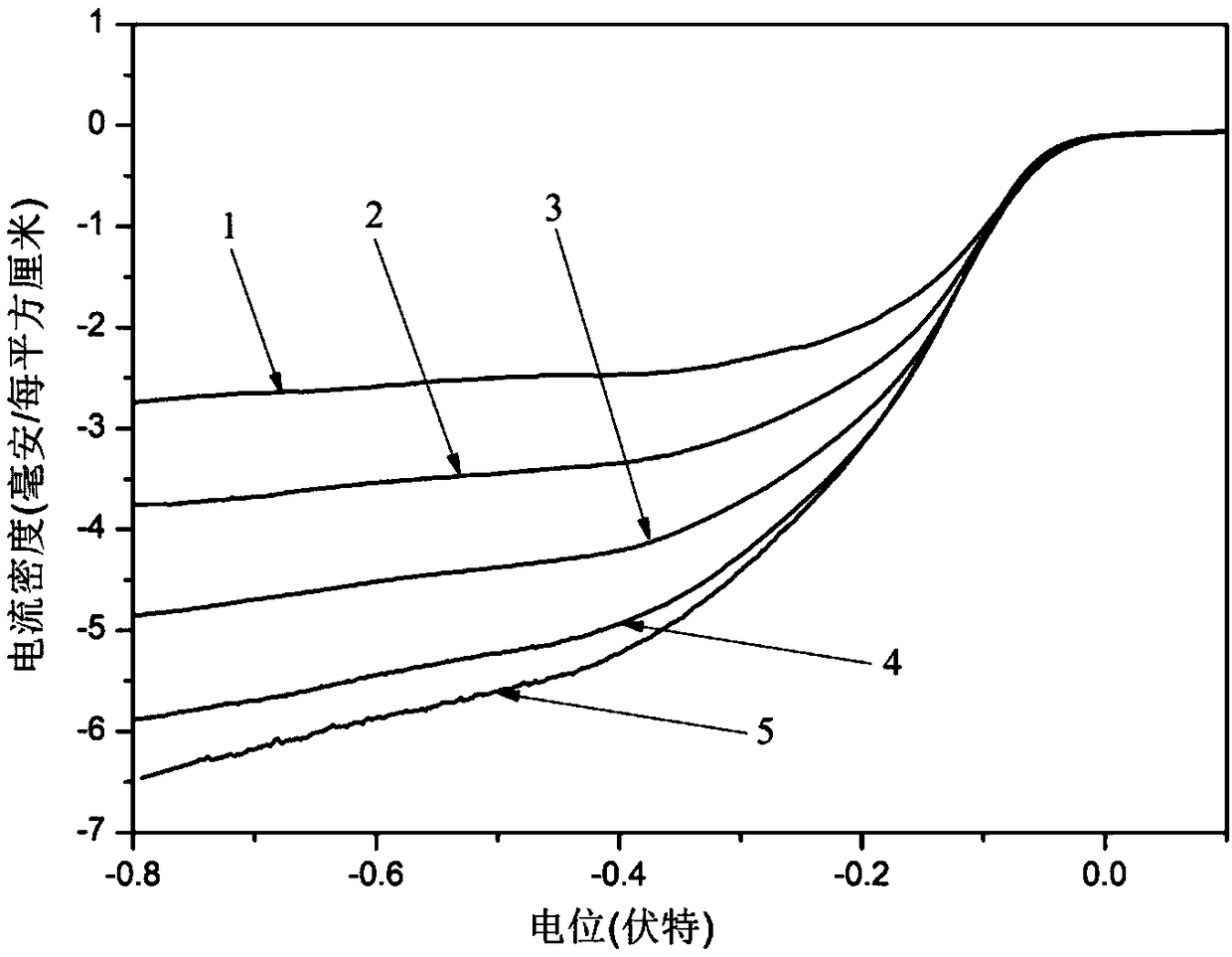
Ag-CuO-NrGO air electrode with supercapacitor performance and preparation method
An air electrode and supercapacitor technology, applied to battery electrodes, fuel cell half-cells, primary battery half-cells, electrical components, etc., can solve the problems of easy falling off of the catalyst layer and low efficiency of zinc-air batteries, and achieve Effect of improving charge-discharge cycle efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This example is a CuO-NrGO catalyst layer supported by nickel foam, wherein the ratio of CuO and NrGO is 16:84. Mix 13 mL of graphene oxide and 27 mL of deionized water and stir for 1 hour, put in an oil bath and stir for 10 minutes, then add 5 mL of NH 3 ·H 2 O, stir and keep the temperature at 80°C for 24 hours. Centrifuge and wash multiple times with deionized water to remove remaining NH 3 ·H 2 O, the resulting product is nitrogen-doped reduced graphene oxide NrGO (the NrGO catalyst can be obtained by freeze-drying it). Afterwards, it was dispersed in deionized water and ultrasonicated for 30 minutes to obtain a uniformly dispersed suspension. 1.6 mL of 0.1M CuCl 2 The aqueous solution was added dropwise to the NrGO suspension and stirred for 10 minutes, then 6 mL of 0.1 M KOH was added dropwise and stirred for 1 hour. The suspension was centrifuged and washed with deionized water several times, followed by freeze-drying to obtain the CuO-NrGO catalyst. Put t...
Embodiment 2
[0036] This example is an air electrode prepared by foaming nickel to support an Ag-CuO-NrGO catalyst layer, wherein the ratio of Ag-CuO to NrGO is 9:91. Mix 13 mL of graphene oxide and 27 mL of deionized water and stir for 1 hour, put in an oil bath and stir for 10 minutes, then add 5 mL of NH 3 ·H 2 O, stir and keep the temperature at 80°C for 24 hours. Centrifuge and wash multiple times with deionized water to remove remaining NH 3 ·H 2 O, the resulting product is nitrogen-doped reduced graphene oxide NrGO (the NrGO catalyst can be obtained by freeze-drying it). Afterwards, it was dispersed in deionized water and ultrasonicated for 30 minutes to obtain a uniformly dispersed suspension. 1.6mL of 0.05M AgNO 3 -Cu(NO 3 ) 2 The aqueous solution was added dropwise to the NrGO suspension and stirred for 10 minutes, then 6 mL of 0.1 M KOH was added dropwise and stirred for 1 hour. The Ag-CuO-NrGO catalyst was obtained by centrifuging the suspension and washing it several t...
Embodiment 3
[0040] This example is an air electrode prepared by foaming nickel to support an Ag-CuO-NrGO catalyst layer, wherein the ratio of Ag-CuO to NrGO is 16:84. Mix 13 mL of graphene oxide and 27 mL of deionized water and stir for 1 hour, put in an oil bath and stir for 10 minutes, then add 5 mL of NH 3 ·H 2 O, stir and keep the temperature at 80°C for 24 hours. Centrifuge and wash multiple times with deionized water to remove remaining NH 3 ·H 2 O, the resulting product is nitrogen-doped reduced graphene oxide NrGO (the NrGO catalyst can be obtained by freeze-drying it). Afterwards, it was dispersed in deionized water and ultrasonicated for 30 minutes to obtain a uniformly dispersed suspension. 1.6mL of 0.1M AgNO 3 -Cu(NO 3 ) 2 The aqueous solution was added dropwise to the NrGO suspension and stirred for 10 minutes, then 6 mL of 0.1 M KOH was added dropwise and stirred for 1 hour. The Ag-CuO-NrGO catalyst was obtained by centrifuging the suspension and washing it several t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com