Methyl gallate analogue containing amide structure and application
A technology for methyl gallate and analogs, which is applied in the field of methyl gallate analogs and their preparation, can solve the problem that the efficacy and function of MG are not yet determined, and achieve the effects of good inhibitory activity and good anti-inflammatory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
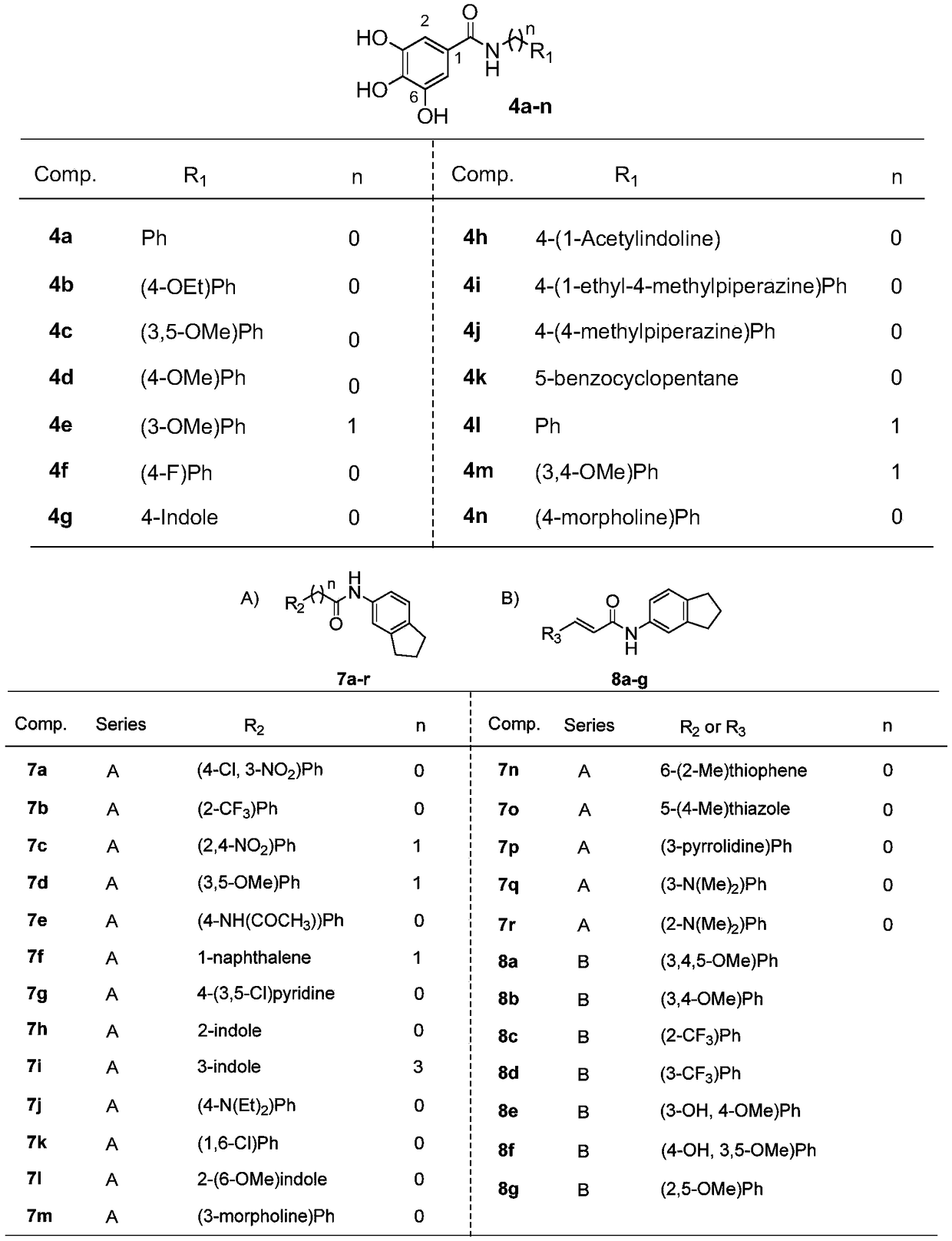
[0045] The chemical structure characterization data of the compound 4a synthesized in Example 1
[0046] Compound (4a): 3,4,5-Trihydroxy-N-phenylbenzamide
[0047] 3,4,5-trihydroxy-N-phenylbenzamide
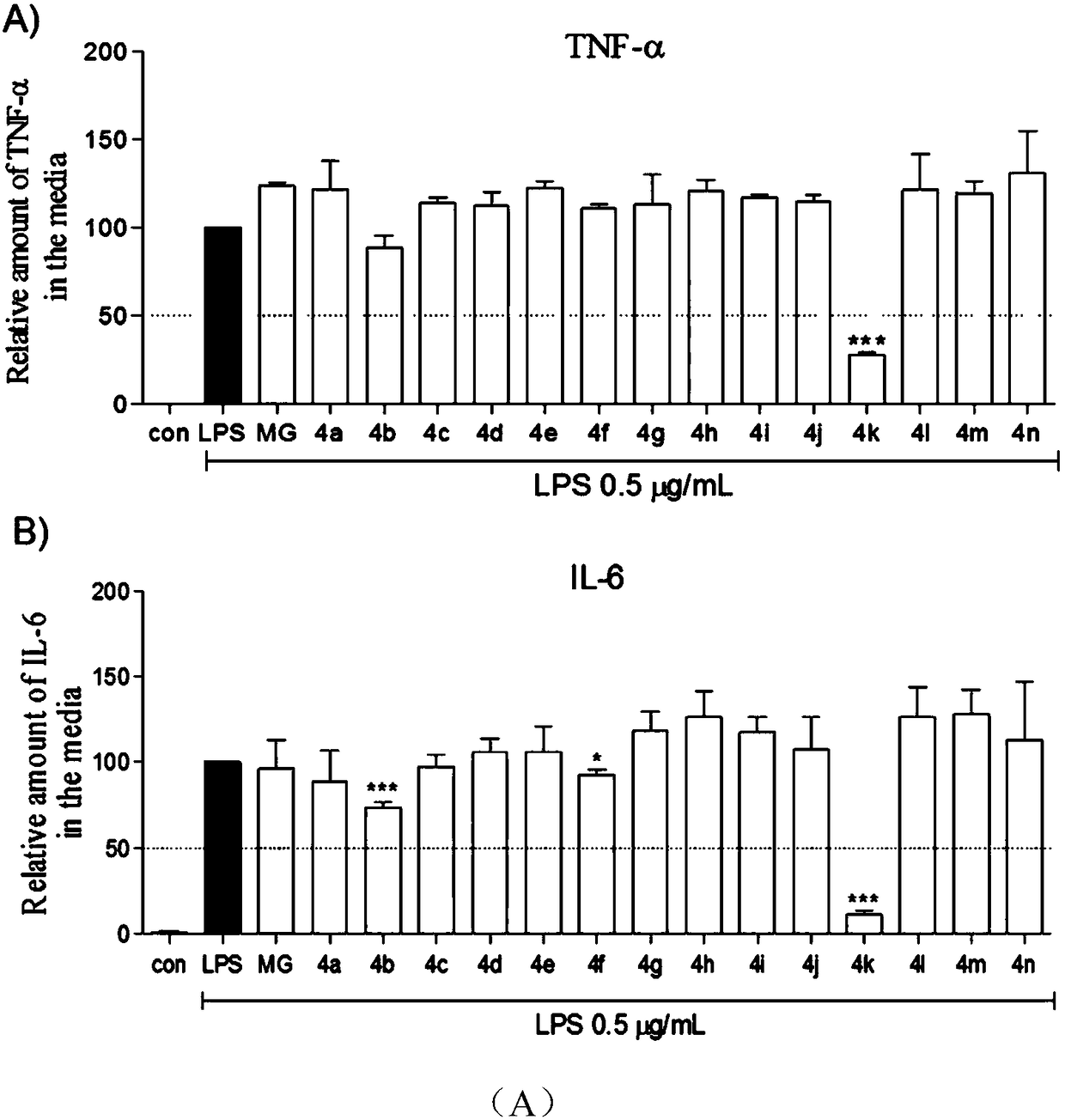
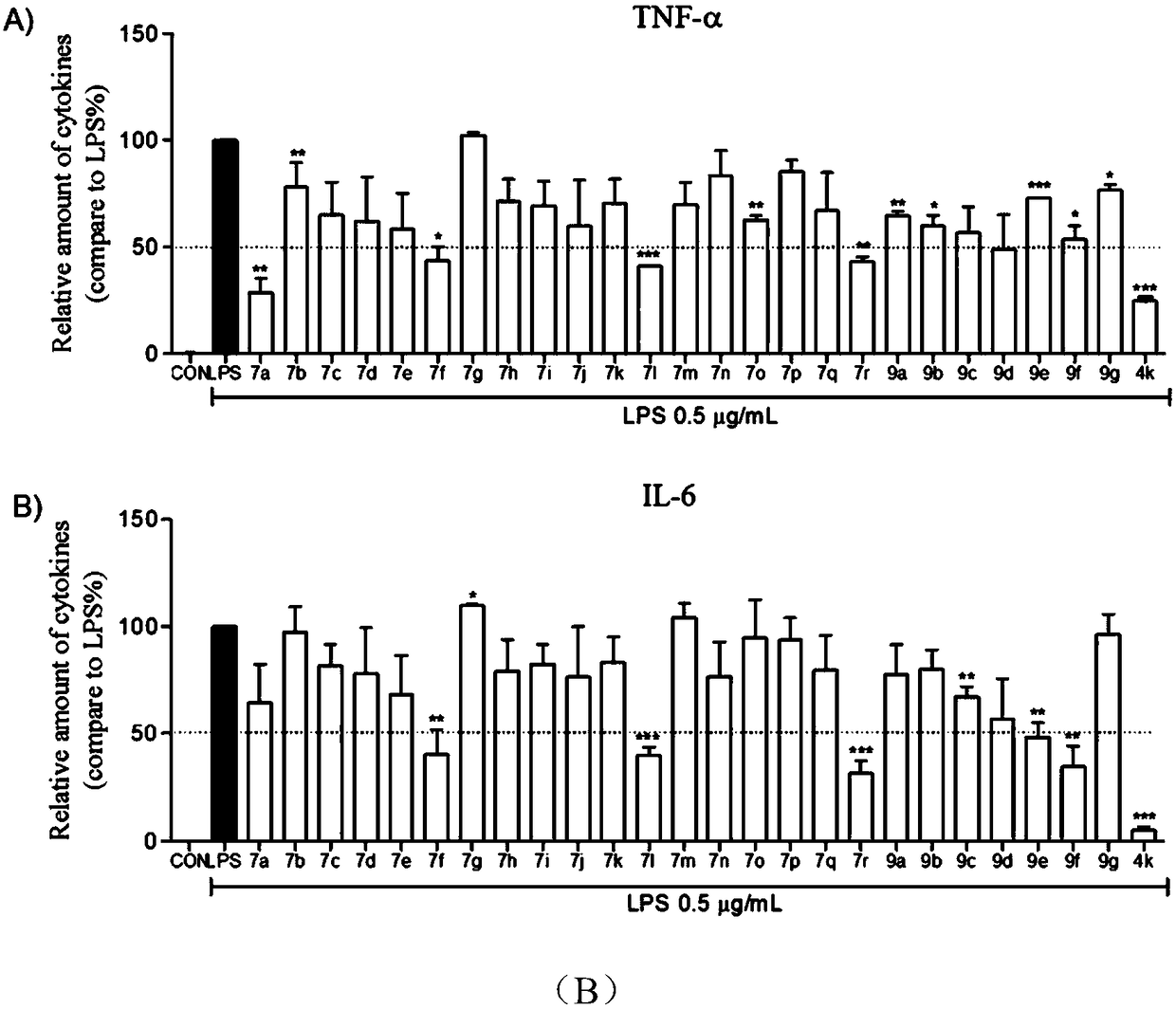
[0048] White powder, yield: 48.8%, melting point: 203.6-206.0.3°C. 1 H NMR (500MHz, DMSO-d 6 )δ: 9.90 (1H, s, Ar-NH), 9.08 (3H, s, Ar-OH), 7.74 (2H, d, J=7.864Hz, ArH 2 ), 7.31 (2H, t, J=7.830Hz, ArH 2 ), 7.05 (1H, t, J=7.351Hz, ArH), 6.95 (2H, s, ArH 2 ). 13 C NMR (125MHz, DMSO-d 6 )δ(ppm): 165.54, 145.44×2, 139.55, 136.71, 128.43×2, 124.99, 123.06, 120.09×2, 107.15×2. ESI-MS m / z: 246.09(M+H) + .Such as figure 1 Shown is the chemical structure of a class of methyl gallate analogues synthesized by the present invention with the hydrolyzed methyl gallate ester bond as the core structure.
Embodiment 2
[0049] The chemical structure characterization data of the compound 4b synthesized in Example 2
[0050] Compound (4b): N-(4-ethoxyphenyl)-3,4,5-trihydroxybenzamideN-(4-ethoxyphenyl)-3,4,5-trihydroxybenzamide
[0051] White powder, yield: 44.7%, melting point: 191.2-194.1°C. 11 H NMR (500MHz, DMSO-d 6 )δ: 9.76 (1H, s, Ar-NH), 9.13 (2H, s, Ar-OH), 8.79 (1H, s, Ar-OH), 7.61 (2H, d, J=8.429Hz, ArH 2 ),6.92(2H,s,ArH 2 ), 6.86 (2H, d, J=8.439Hz, ArH 2 ),3.98-3.95(2H,m,Ar-OCH 2 -), 1.31 (3H, t, J=6.640Hz, Ar-OCH 2 -CH 3 ). 13 C NMR (125MHz, DMSO-d 6 )δ(ppm): 165.12, 154.37, 145.41×2, 136.48, 132.54, 125.14, 121.64×2, 114.08×2, 107.02×2, 62.99, 14.67. ESI-MS m / z: 290.10(M+H) + .Such as figure 1 Shown is the chemical structure of a class of methyl gallate analogues synthesized by the present invention with the hydrolyzed methyl gallate ester bond as the core structure.
Embodiment 3
[0052] The chemical structure characterization data of the compound 4c synthesized in embodiment 3
[0053] Compound (4c): N-(3,5-dimethoxyphenyl)-3,4,5-trihydroxybenzamide
[0054] N-(3,5-dimethoxyphenyl)-3,4,5-trihydroxybenzamide
[0055] Gray powder, yield: 49.3%, melting point: 129.7-131.8°C. 1 H NMR (500MHz, DMSO-d 6 )δ: 9.81 (1H, s, Ar-NH), 9.19 (3H, s, Ar-OH), 7.07 (2H, d, ArH 2 ),6.93(2H,s,ArH 2 ), 6.21(1H, t, J=2.008Hz, ArH), 3.71(6H, s, Ar-OCH 3 ). 13 C NMR (125MHz, DMSO-d 6 )δ(ppm): 165.56, 160.25×2, 145.44×2, 141.25, 136.78, 124.92, 107.13×2, 98.20×2, 95.15, 55.00×2. ESI-MS m / z: 306.05(M+H) + .Such as figure 1 Shown is the chemical structure of a class of methyl gallate analogues synthesized by the present invention with the hydrolyzed methyl gallate ester bond as the core structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com