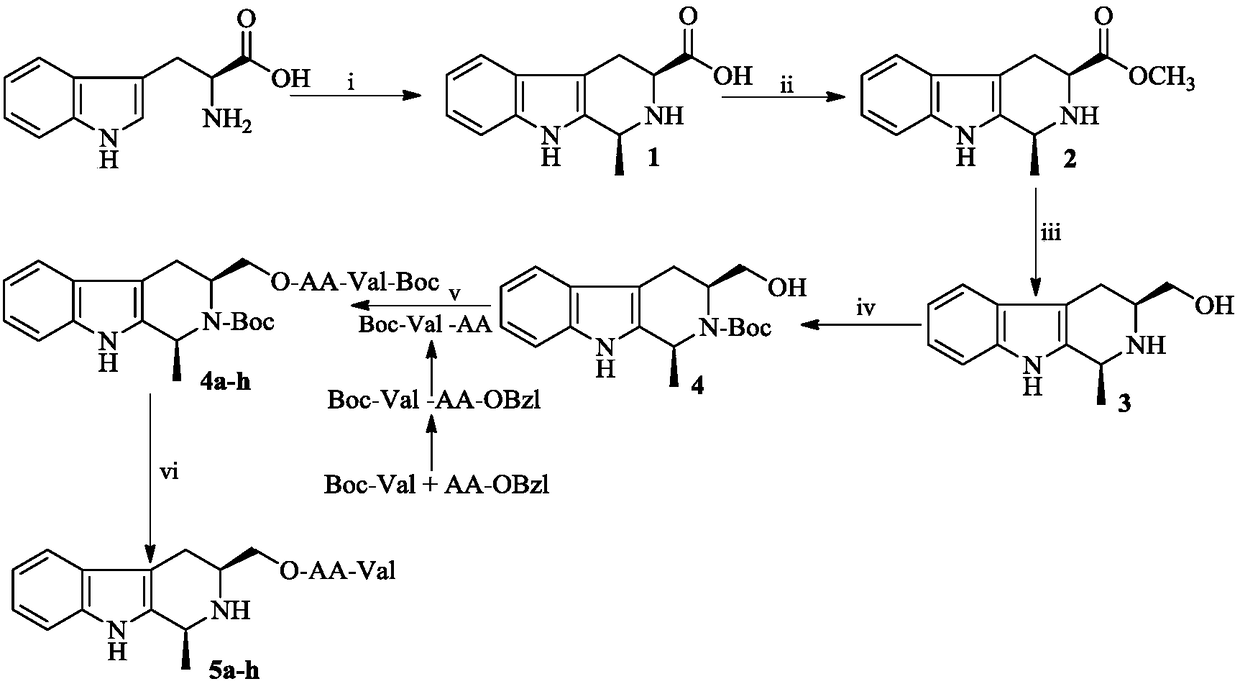
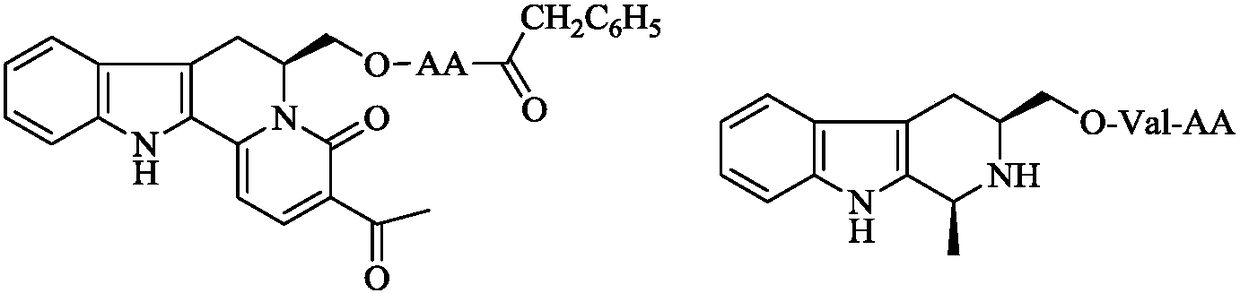
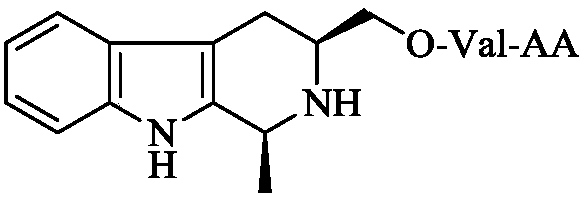
Dipeptide modified 1-methyl-3-hydroxymethyl-tetrahydro-beta-carboline, synthesis and applications thereof
A technology of hydroxymethyl, boc-aa-val-obzl, applied in the preparation of anti-tumor drugs and P-selectin inhibitors, anti-tumor activity, the active field of inhibiting the expression of P-selectin, can solve the problem of P-selectin -Selectin is not inhibited etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of (1S,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid
[0019] Slowly drop 0.2 mL of concentrated sulfuric acid (98%) into the suspension of 5.0 g (24.5 mmol) of L-tryptophan and 400 mL of distilled water. After ultrasonically dissolving everything, 4.5 mL (49.0 mmol) of acetaldehyde (40%) was added dropwise. The reaction mixture was stirred at room temperature for 12 h, adjusted to pH 7 with concentrated ammonia water, and allowed to stand for 1 h. The reaction mixture was filtered, the filter cake was thoroughly washed with distilled water, and dried to obtain 4.51 g (80%) of the title compound as a colorless powder. ESI-MS(m / z):231[M+H] + ; 1 H NMR (300MHz, DMSO-d6): δ (ppm) = 10.99 (s, 1H), 9.18 (s, 1H), 7.44 (d, J = 7.5Hz, 1H), 7.33 (t, J = 8.0Hz, 1H),7.08(t,J=8.0Hz,1H),6.99(t,J=7.5Hz,1H),4.22(q,J=4.8Hz,1H),3.69(dd,,J=10.5Hz,J =5.0Hz, 1H), 3.14(dd, J=10.5Hz, J=2.4Hz, 1H), 2.83(ddd, J=10.5Hz, J=5.0Hz, J=2.4Hz, 1H), 1.38 (d, ...
Embodiment 2
[0020] Example 2 Preparation of (1S,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester
[0021] Slowly drop 5mL of thionyl chloride into 100mL of methanol under ice-water bath and stir for 30min. Add 2.0 g (8.7 mmol) (1S,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid to the resulting solution, and stir at room temperature for 14 h. Hydrogen chloride was removed under reduced pressure, and the reaction mixture was washed with saturated NaHCO 3 The pH of the aqueous solution was adjusted to 7. Let it stand fully, and dissolve the filtered precipitate with 100 mL of ethyl acetate. The resulting solution was sequentially washed with saturated NaHCO 3 Wash with aqueous solution (30mL×3) and saturated NaCl aqueous solution (30mL×3). Ethyl acetate phase with Na 2 SO 4 Dry for 8 h, filter, and concentrate the filtrate under reduced pressure to afford 1.35 g (65%) of the title compound. ESI-MS(m / z): 245[M+H] + ; IR (cm -1 ):3202,2970,2937...
Embodiment 3
[0022] Example 3 Preparation of (1S,3S)-1-methyl-3-hydroxymethyl-1,2,3,4-tetrahydro-β-carboline
[0023] 1.0 g (4.10 mmol) of (1S,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester was dissolved in 30 mL of anhydrous tetrahydrofuran. 0.15 g (12.30 mmol) lithium aluminum hydride was added to the solution within 50 min under an ice-water bath. The reaction mixture was stirred at room temperature for 4 h, quenched by adding 0.5 mL aqueous sodium hydroxide solution (10%) and stirring at room temperature for 1 h. Filter and wash the filter cake repeatedly with anhydrous tetrahydrofuran. The collected filtrates were concentrated under reduced pressure to afford 0.50 g (48%) of the title compound. ESI-MS(m / z):217[M+H] + ; IR (cm -1 ):3202,2970,2937,2452,1750,1682,1559,1500,1457,1320,1237,1149,1015; 1 H NMR (300MHz, DMSO-d6): δ (ppm) = 10.72 (s, 1H), 7.34 (d, J = 7.8Hz, 1H), 7.28 (d, J = 7.8Hz, 1H), 7.01 (t, J=13.8Hz, 1H), 6.95(t, J=15.9Hz, 1H), 4.74(t, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com