Preparation method of porous lanthanum oxide
A technology of lanthanum oxide and lanthanum salt, which is applied in the field of preparation of porous lanthanum oxide, can solve problems such as harsh conditions, expensive raw materials, and difficult operation and control, and achieve the effect of mild conditions and simple and easy-to-operate preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 0.1g of glycerol and 0.44g of La (NO 3 ) 3 ·6H 2 O, be dissolved in 2ml distilled water respectively, drip the polyethylene glycol of 0.21ml 0.01mol / L in two kinds of solutions, then add a certain amount of ionic liquid to the glycerol solution, adjust the mixed solution with concentrated sulfuric acid and ammoniacal liquor PH, and then stir uniformly with a magnetic heating stirrer, then heat in a muffle furnace for a certain period of time to obtain the porous lanthanum oxide sample synthesized under the reaction conditions.
[0035] In order to determine the best experimental conditions, an orthogonal experimental table is established on the premise of five factors and four levels. The five factors are pH value, initial mass concentration, dosage, temperature, and adsorption time, and four levels are selected for the five factors. Orthogonal experiment, the factors and levels of the orthogonal experiment are shown in Table 1, and the orthogonal experiment tab...
Embodiment 2
[0042] The effect of ionic liquid concentration on the product
[0043] The reaction conditions in Example 1 were fixed: the pH of the solution was 2, the calcination temperature was 750° C., and the calcination time was 1.5 h.
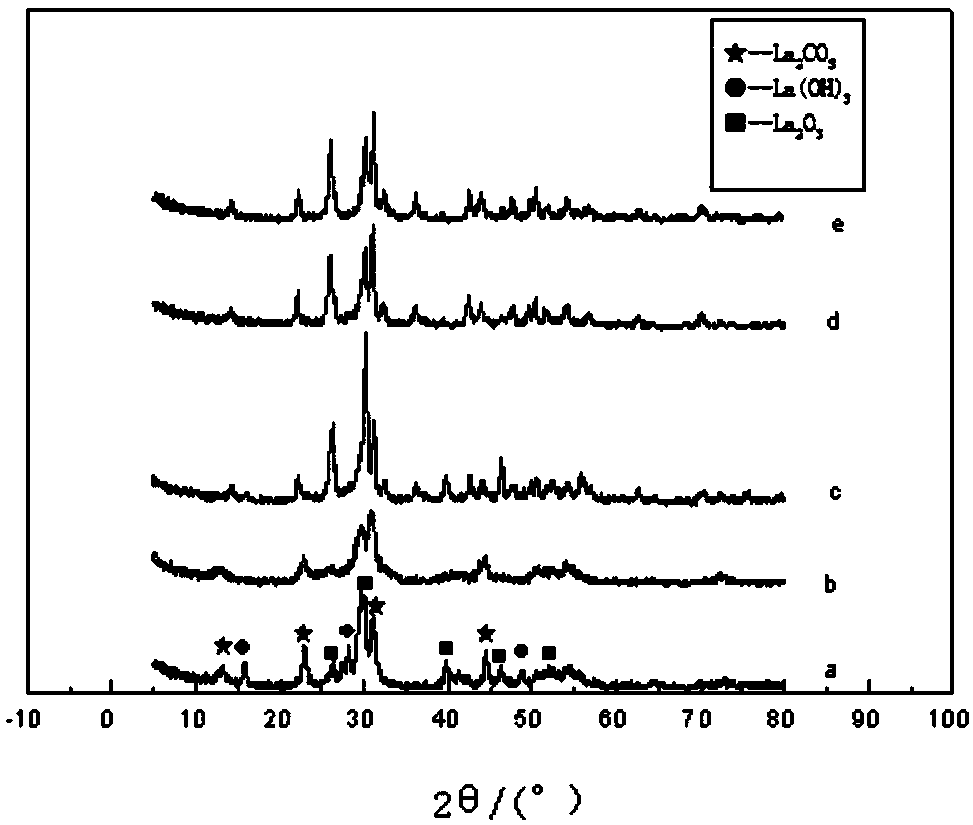
[0044] XRD characterization as figure 1 , figure 1 (a) 0.01g / ml; (b) 0.02g / ml; (c) 0.03g / ml; (d) 0.04g / ml; (e) 0.05g / ml. 2 O 3 After comparing with the standard cards, we can see that the prepared porous lanthanum oxide is of hexagonal crystal form. from figure 1 It can be seen that when the concentration of ionic liquid is 0.03g / ml, the crystallinity of the prepared sample is the best and the purity is the highest. Therefore, the ionic liquid concentration of 0.03 g / ml is selected as the appropriate concentration for preparing the product. Estimated by Scherrer's formula D=Kλ / Bcosθ, the average size of the product prepared at this ionic liquid concentration is 20.8 nm.
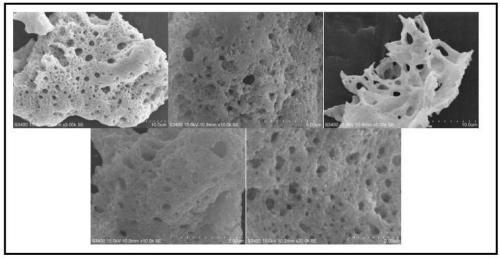
[0045] SEM characterization such as figure 2 , the concentrations in th...
Embodiment 3
[0052] The effect of pH on the product
[0053] Do five groups of comparative experiments, so that the pH of these five groups of experiments are 1, 2, 3, 4, and 5 respectively. The reaction steps are as in Example 1, the concentration of the fixed ionic liquid is 0.03, the heating temperature is 750 degrees, and the heating time is 1.5h , other reaction conditions remain unchanged, the porous lanthanum oxide samples prepared under the pH environment were synthesized through experiments, and the effect of different pH on the reaction was explored through characterization, and the optimal pH value was selected. The difference of pH value in the solution directly affects the particle size of the product. In the reaction of preparing porous lanthanum oxide by combustion method, glycerol not only acts as a combustion agent, but also complexes metal ions. The ions are evenly distributed in the complex. When the pH value is too small, the complexation of the lanthanum ions is not st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap