Chimeric antigen recipient cell taking ROBO1 as target, preparation and application thereof
A chimeric antigen receptor, antigen technology, applied in receptor/cell surface antigen/cell surface determinant, genetically modified cells, for targeting specific cell fusion, etc., can solve the problem of low tumor cell specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
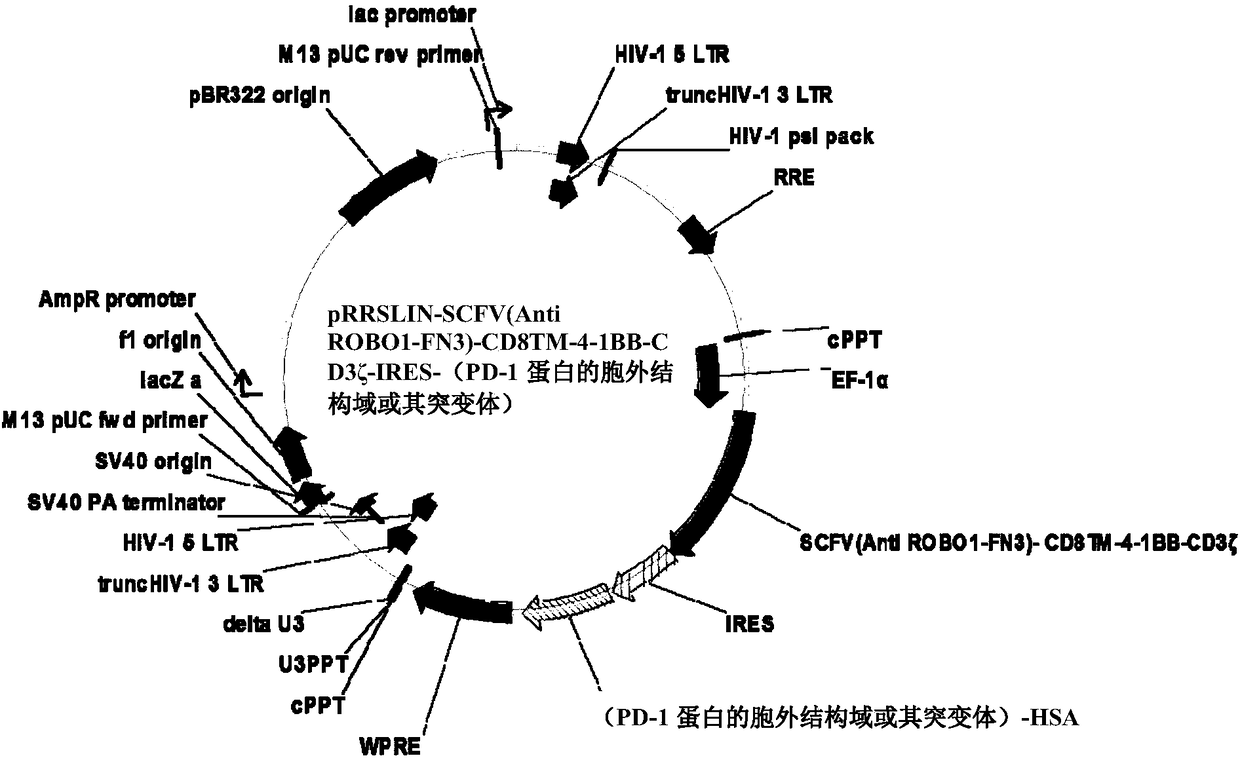
[0115] Example 1 Preparation of lentiviral expression vector
[0116] According to "Engineering high-affinity PD-1 variants for optimized immunotherapy and immune-PET imaging" (Maute RL, Gordon SR, Mayer AT, etc. Proceedings of the National Academy of Sciences, 2015, 112(47): E6506-E6514.) The HAC sequence information of the PD-1 mutant molecule, the synthesis of the extracellular domain of the PD-1 protein or its mutant gene sequence, as shown in SEQ ID NO:1; search and synthesize the known human anti-ROBO1 from the GenBank database. FN3scFv (hereinafter referred to as scFv) gene sequence, IRES internal ribosome entry site, human CD8 transmembrane region gene sequence, human 4-1BB intracellular region gene sequence, CD3ζ intracellular region gene sequence, respectively, as SEQ ID NO: 2 -6 shown.
[0117] The sequence of the above-mentioned genes was sequenced according to scFv gene, CD8 gene, human 4-1BB intracellular region gene and CD3ζ intracellular region gene, IRES internal ...
Embodiment 2
[0119] Example 2 Preparation of Lentivirus
[0120] 1. 24 hours before transfection, about 8×10 per dish 6 The 293T cells were seeded into a 15cm petri dish. Ensure that the cells are at about 80% confluence and are evenly distributed in the culture dish during transfection.
[0121] 2. Prepare solution A and solution B
[0122] Solution A: 6.25mL 2×HEPES buffer (5 large dishes are used to pack together, the best effect).
[0123] Solution B: Add the following plasmid mixture: 112.5μg scFv-CD8TM-4-1BB-CD3ζ-IRES- (the extracellular domain of PD-1 protein or its mutant) (target plasmid); 39.5μg pMD2.G( VSV-Genvelop); 73μg pCMVR8.74 (gag, pol, tat, rev); 625μL 2M calcium ion solution. Total volume of solution A: 6.25 mL.
[0124] 3. Mix solution B thoroughly, add solution A dropwise while vortexing solution A gently, and let stand for 5-15 minutes. Gently vortex the above-mentioned mixed solution of A and B, add dropwise to the petri dish containing 293T cells, and gently shake the pet...
Embodiment 3
[0127] Example 3 Preparation of CAR-T cells
[0128] Follow the steps in Example 3 of CN201610226230.9 to prepare CAR-T cells.
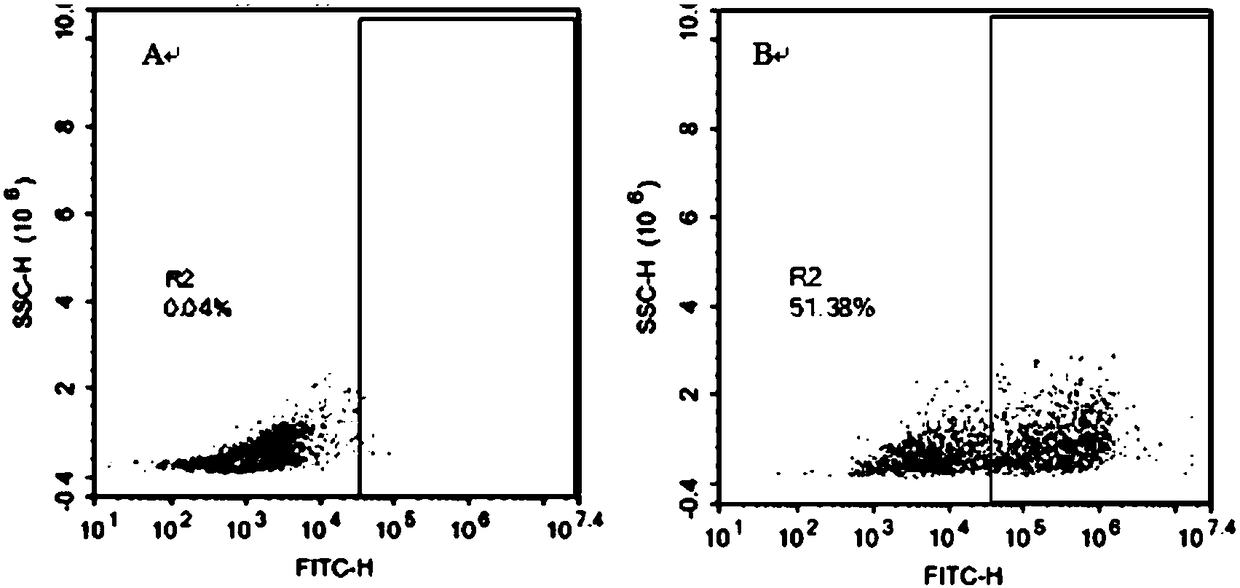
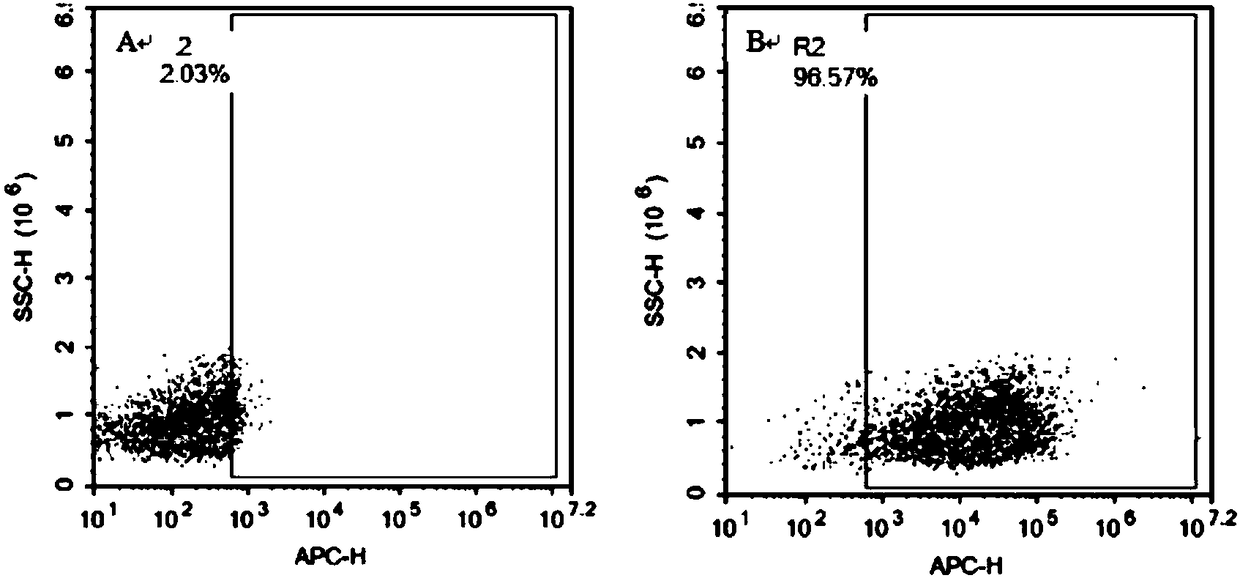
[0129] After virus infection, use flow cytometry to detect CAR-T positive rate. The flow cytometry diagram is as follows figure 2 As shown, the results showed that the positive rate was 51.38%, and CAR-T cells were successfully prepared. ( figure 2 : Flow cytometry detects the positive expression rate of CAR-T, figure 2 -A is T cell that has not been infected by virus, as a control; figure 2 -B is T cell after virus infection. The antibody is fluorescently labeled with FITC and is indicated on the abscissa. If the T cell successfully expresses the CAR molecule, the signal value will increase significantly. )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com