Symmetric inverse z-type photocatalyst and preparation method and application thereof
A photocatalyst, symmetrical technology, applied in the field of photocatalysis, which can solve the problems of long period and no formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
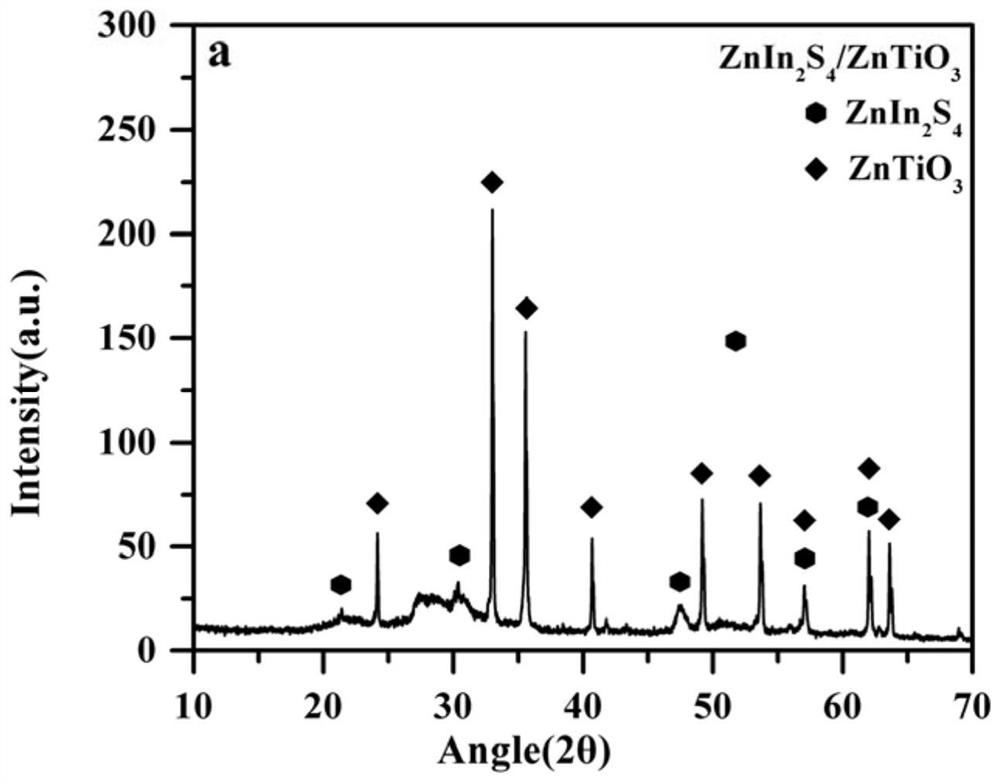
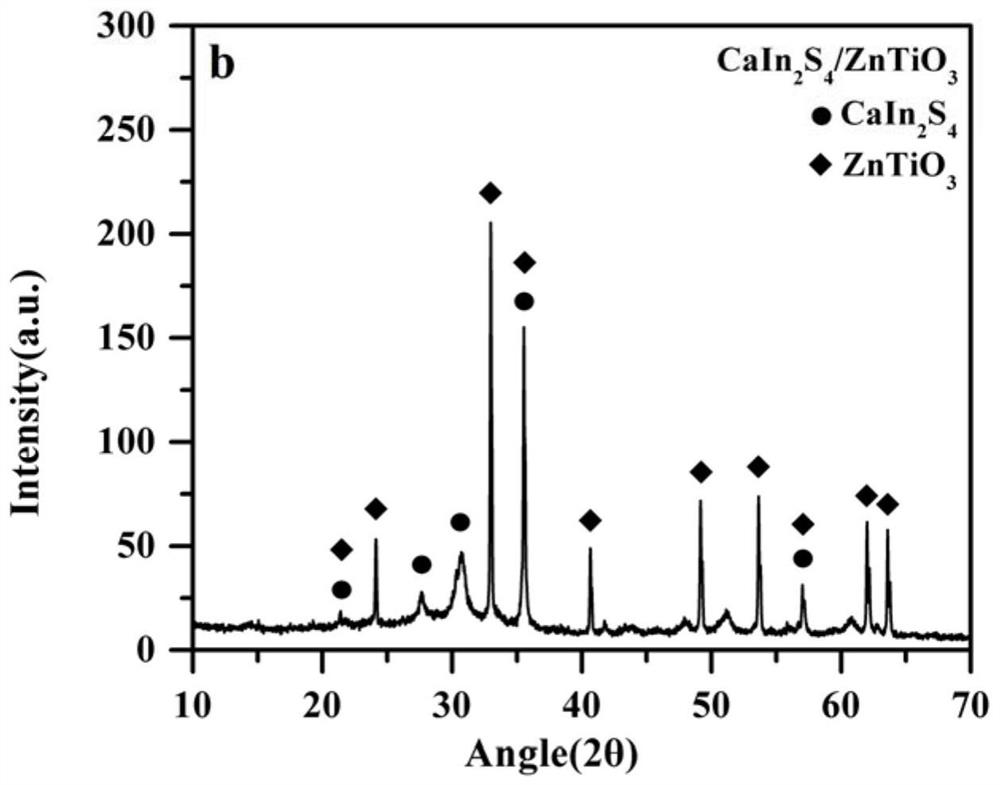
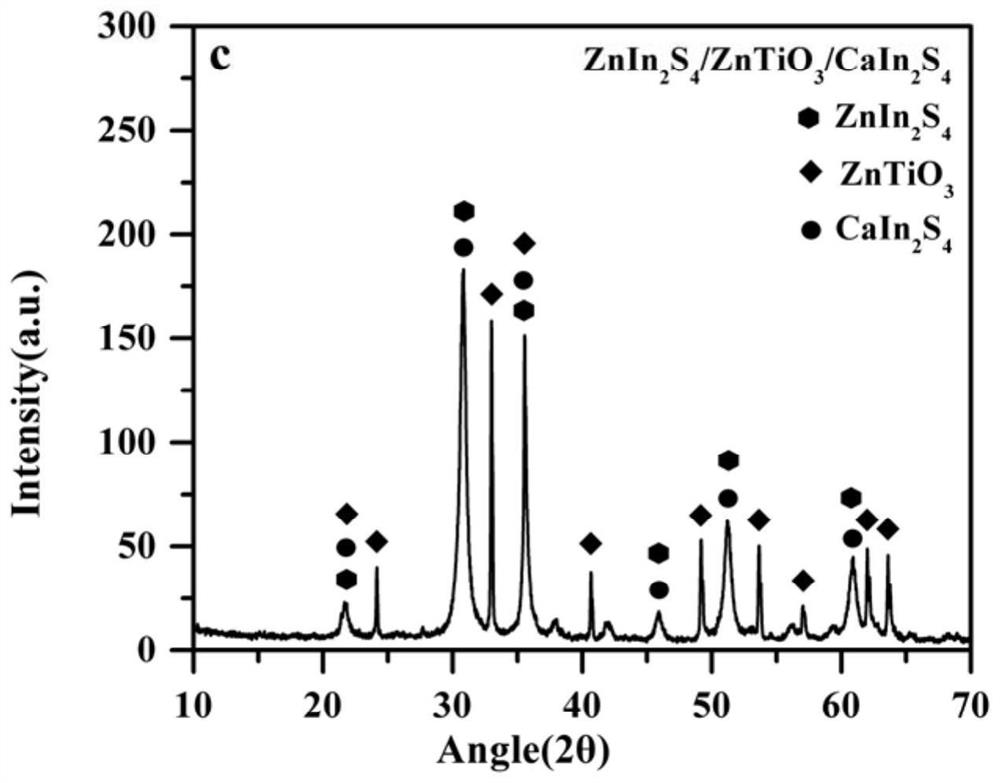
[0020] Example 1 Symmetric inverse Z-type photocatalyst ZnIn 2 S 4 / Er 3+ :Y 3 Al 5 o 12 @ZnTiO 3 / CaIn 2 S 4
[0021] The preparation method is as follows:
[0022] 1. ZnIn 2 S 4 Preparation of:
[0023] ZnCl 2 (0.68g) and In(NO 3 ) 3 (3.0g) was dissolved in 80mL of deionized water, then thioacetamide (2.3g) was added, stirred on a magnetic ion stirrer for 1.0h to form a transparent solution. Then the formed transparent solution was transferred to a polytetrafluoroethylene-lined stainless steel reactor and heat-treated at 160 °C for 6.0 h. The obtained product was cooled to room temperature, and then washed with deionized water and ethanol. Finally, the obtained product was put into an oven, dried at 80° C. for 6.0 h, and ground.
[0024] 2. CaIn 2 S 4 Preparation of:
[0025] Ca(NO 3 ) 2 4H 2 O(1.2g), In(NO 3 ) 3 (3.0g) was dissolved in 80mL deionized water, stirred with magnetic force at room temperature until completely dissolved, then added thioac...
Embodiment 2
[0035] Example 2 Photocatalytic Degradation of Acid Orange II by Symmetric Inverse Z-type Photocatalyst
[0036] Simulated sunlight photocatalytic degradation: Measure 25mL of 10mg / L Acid Orange II solution in a 100mL Erlenmeyer flask, add 25mg of the catalyst of the present invention and comparative example prepared in Example 1 as shown in Table 1, and irradiate 6.0 mg under simulated sunlight h. Filter and measure its UV spectrum at 200-800nm. Take the absorbance at 485nm to calculate the degradation rate of Acid Orange II, and the results are shown in Table 1.
[0037] Degradation rate (%) = (C 0 –C) / C 0 ×100% (where C 0 : the concentration of the stock solution; C: the concentration of the sample)
[0038] (1) Effect of light time on degradation rate
[0039] Table 1 ZnIn 2 S 4 / Er 3+ :Y 3 Al 5 o 12 @ZnTiO 3 / CaIn 2 S 4 Photocatalytic degradation of acid orange Ⅱ by simulated sunlight
[0040]
[0041] As shown in Table 1, with the prolongation of the l...
Embodiment 3
[0047] Example 3 Symmetric inverse Z-type photocatalyst photocatalytic hydrogen production with Acid Orange II as sacrificial agent
[0048] Method: Measure 500 mL of 50 mg / L Acid Orange II solution in a photocatalytic hydrogen production reactor, add 500 mg of the catalysts of the present invention and comparative examples prepared in Example 1 as shown in Table 1, and irradiate with visible light for 6.0 h. Gas chromatography was used to measure the amount of hydrogen generated during the reaction. The results are shown in Table 3.
[0049] (1) Effect of light time on hydrogen production
[0050] Table 3 Symmetric inverse Z-type photocatalyst simulated sunlight photocatalytic hydrogen production
[0051]
[0052] As shown in Table 3, with the prolongation of the light time, the hydrogen production of the four catalysts increased with time, and the hydrogen production showed an increasing trend. Among them, when the light time is 6.0h, ZnIn 2 S 4 / Er 3+ :Y 3 Al 5 o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com