A construction method and application of ctla4 gene humanized animal model
An animal model and construction method technology, applied in the construction of CTLA4 gene humanized animal model, the application field of biomedicine, can solve the problems of toxic reaction, limit the research and development and application of anti-human CTLA4 antibody, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
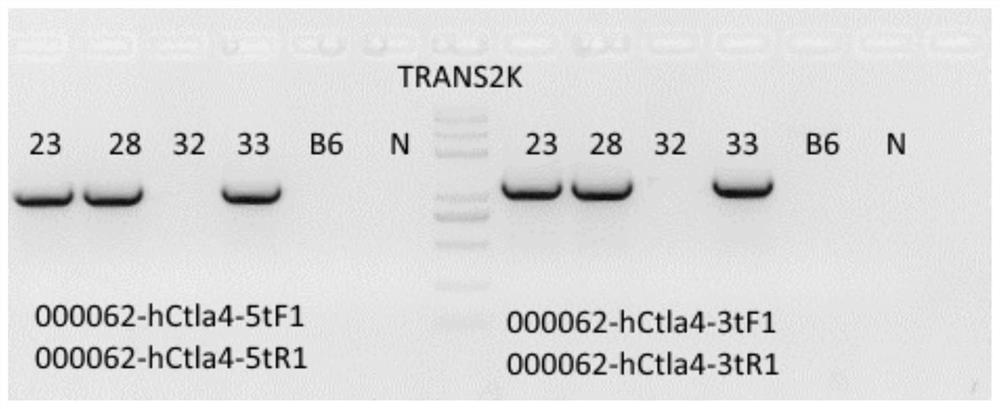
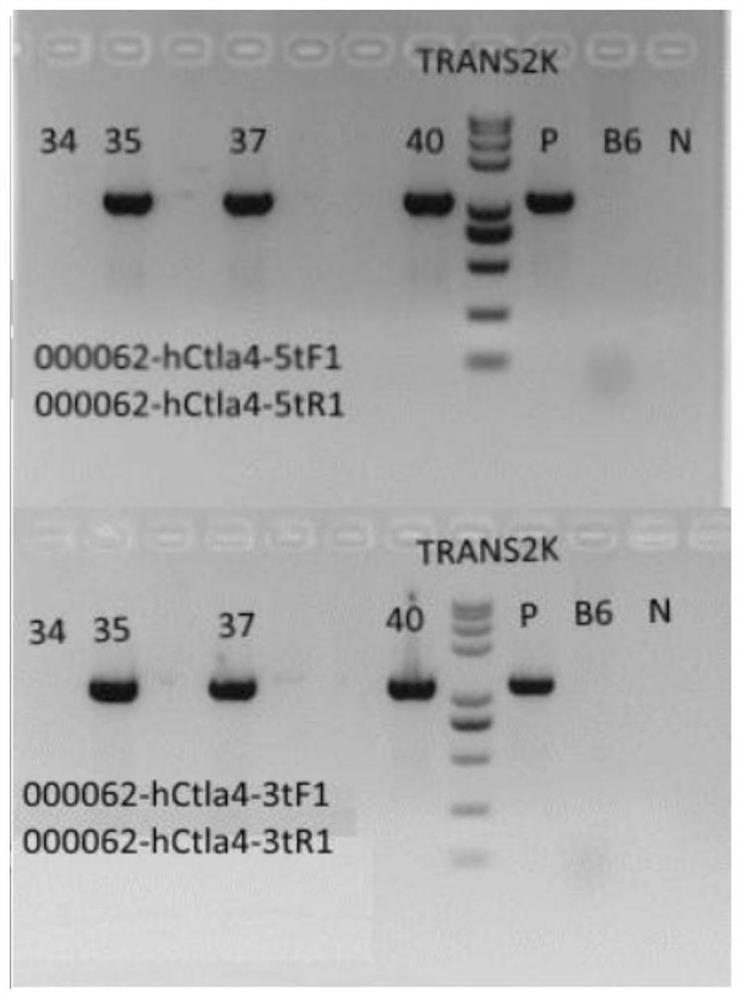
[0048] Example 1: Establishment of CTLA4 humanized mouse model
[0049] Using CRISPR Cas9 to replace Exon2 of the human CTLA4 gene with Exon2 of the mouse Ctla4 gene, a CTLA4 gene humanized mouse model was established. C57BL / 6 mice are currently a relatively mature mouse strain, and C57BL / 6 mice were used as background mice to successfully obtain a CTLA4 humanized mouse model.
[0050] (1) Determine the replacement region of the human fragment and the inserted human sequence
[0051] According to the binding functional domain of human CTLA4 and B7 protein, Exon2 of human CTLA4 sequence was selected to replace Exon2 of mouse Ctla4, and the sequence of mouse Ctla4 signal peptide, transmembrane region and intracellular region was retained. The selected human CTLA4 gene sequence is SEQNo. .1 shown.
[0052] (2) Screening of sgRNA prepared from CTLA4 humanized mice
[0053] Design sgRNA targeting murine sequence in the humanized replacement region. Design and synthesize sgRNA s...
Embodiment 2
[0080] Example 2: Expression and functional verification of CTLA4 humanized mouse model
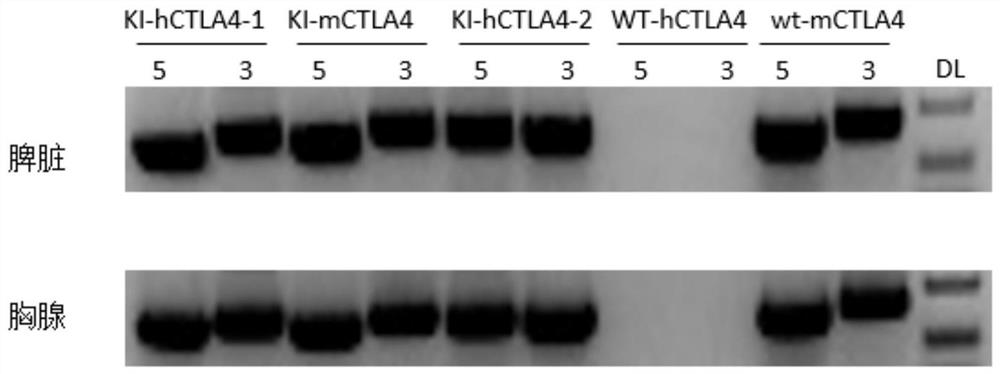
[0081] 1. Verification of mRNA expression in CTLA4 humanized mice
[0082]Detect the transcription of human CTLA4 in heterozygotes by mRNA, and then analyze the expression of humanized CTLA4 gene in heterozygotes and homozygotes by flow cytometry and check the immune cell population. After analysis, CTLA4 humanized mice can be successfully Humanized CTLA4 mice that express the human CTLA4 gene and do not cause obvious abnormalities in the immune system of the mice can be used for subsequent tumor drug efficacy experiments.
[0083] The detection method is as follows: the samples used in the experiment are humanized CTLA4 (KI / wt) and C57BL / 6 wild type (wt / wt) mice. Extract mouse spleen and thymus RNA for RT-PCR, and sequence the PCR products to verify whether the splicing of CTLA4mRNA is correct. Primers (CTLA4-KI-mRNA-F1, CTLA4-KI-mRNA-R1) are located outside the 5-terminus of murine CT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com