Immunosuppressive drug and preparation method thereof
A technology of immunosuppression and drugs, which is applied in the field of immunosuppressive drugs and its preparation, can solve the problems of no immune hypersensitivity diseases, reduce the chance of cross-infection or iatrogenic infection, suppress immune response, and reduce medical operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of immunosuppressive drug of the present invention
[0052] 1. Experimental Materials and Reagents
[0053] The C57BL / 6 mice used in the experiment were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., aged 6-8 weeks, weighing about 18g.
[0054] Fetal calf serum (FCS): purchased from PAN company;
[0055] RPMI-1640 medium: purchased from PAN company;
[0056] The mouse pancreatic stromal cell line Y0501 was preserved for the establishment of this subject;
[0057] Mouse GM-CSF and IL-4 were purchased from Peprotech, USA;
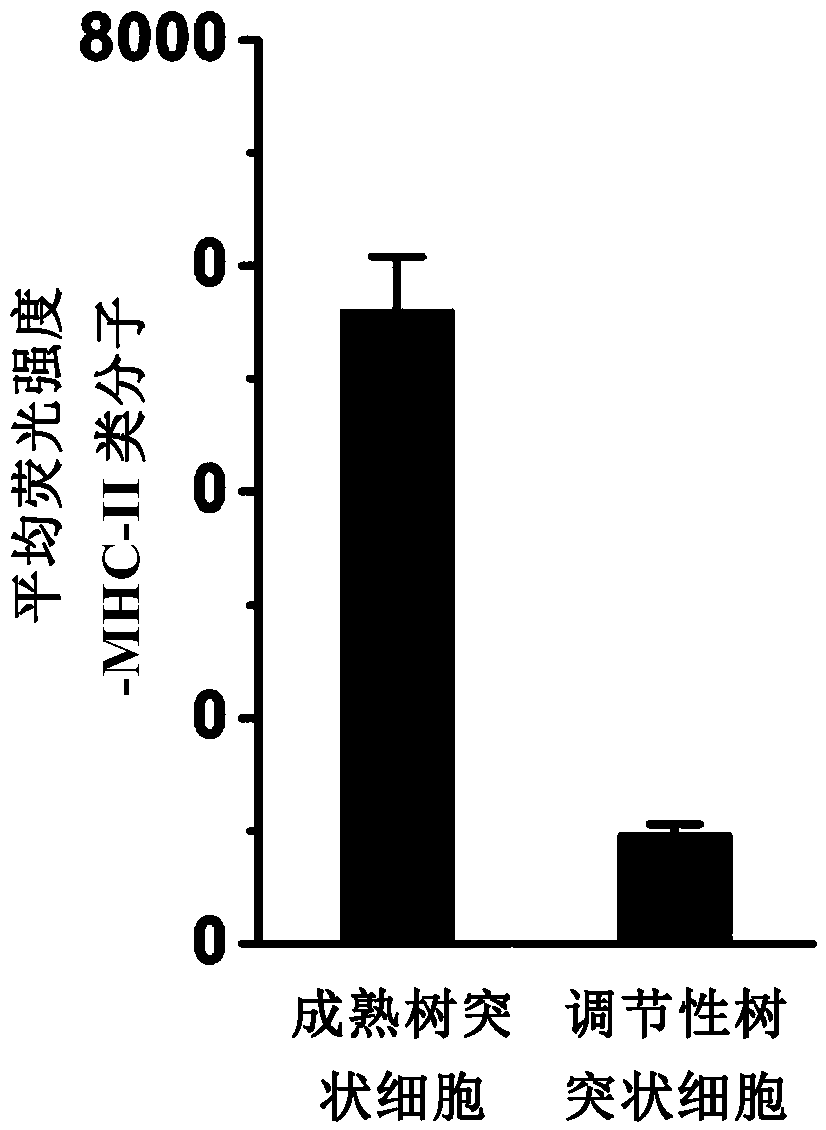
[0058] FITC-labeled anti-mouse MHC-II, FITC-labeled anti-mouse CD40, FITC-labeled anti-mouse CD80, FITC-labeled anti-mouse CD86 and FITC-labeled isotype control antibodies were purchased from Biolegend, USA.
[0059] 2. Experimental steps
[0060] (1) Separation of mouse peripheral blood mononuclear cells with Ficoll separation medium (density 1.083);
[0061] (2) The isolated mouse peripheral blood mon...
Embodiment 2
[0072] Adoptive reinfusion of regulatory dendritic cells in rheumatoid arthritis
[0073] 1. Experimental Materials and Reagents
[0074] Experimental DBA / 1 mice were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., aged 6-8 weeks, weighing about 18 g.
[0075] Chicken type II collagen and complete Freund's adjuvant were purchased from Sigma-Aldrich, USA;
[0076] 2. Experimental steps
[0077] (1) Chicken type II collagen was emulsified in complete Freund's adjuvant to a final concentration of 1 mg / mL;
[0078] (2) On the 0th day, inject 100uL of emulsified type II collagen into the base of the tail of the recipient mice, and inject the recipient mice at the same site at the same dose on the 21st and 42nd days;
[0079] (3) On the 22nd day, the recipient mice were divided into two groups, and one group was injected with 1×10 6 Regulatory dendritic cells (preparation method is the same as in Example 1), and another group is injected with PBS; ...
Embodiment 3
[0084] Adoptive reinfusion of regulatory dendritic cells to treat diabetic model RIP-OVA mice
[0085] 1. Experimental Materials and Reagents
[0086] Experimental C57BL / 6 mice were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., aged 6-8 weeks, weighing about 18g; RIP-OVA mice and OT-I mice were purchased from Jackson Laboratories in the United States, aged 6 -8 weeks, weight about 18g.
[0087] OVA 257-264 Peptides were purchased from Chinese Peptide Company (Hangzhou, China);
[0088] Anti-mouse CD8a magnetic bead antibody was purchased from Miltenyi, Germany;
[0089] Glucose monitors were purchased from Johnson & Johnson under the One Touch brand.
[0090] 2. Experimental steps
[0091] (1) Isolate spleen cells from OT-I mice, and use anti-mouse CD8a magnetic bead antibody from Miltenyi Company to isolate CD8 T cells from OT-I mice; take 1×10 6 OT-I mouse CD8 T cells, add 1×10 5 Mature dendritic cells (cultivated according to the meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com