Reagent and method for removing formaldehyde
A reagent and formaldehyde technology, applied in the field of formaldehyde removal reagents, can solve the problems of low removal rate and long removal time, and achieve the effect of high removal rate, short removal time and long duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
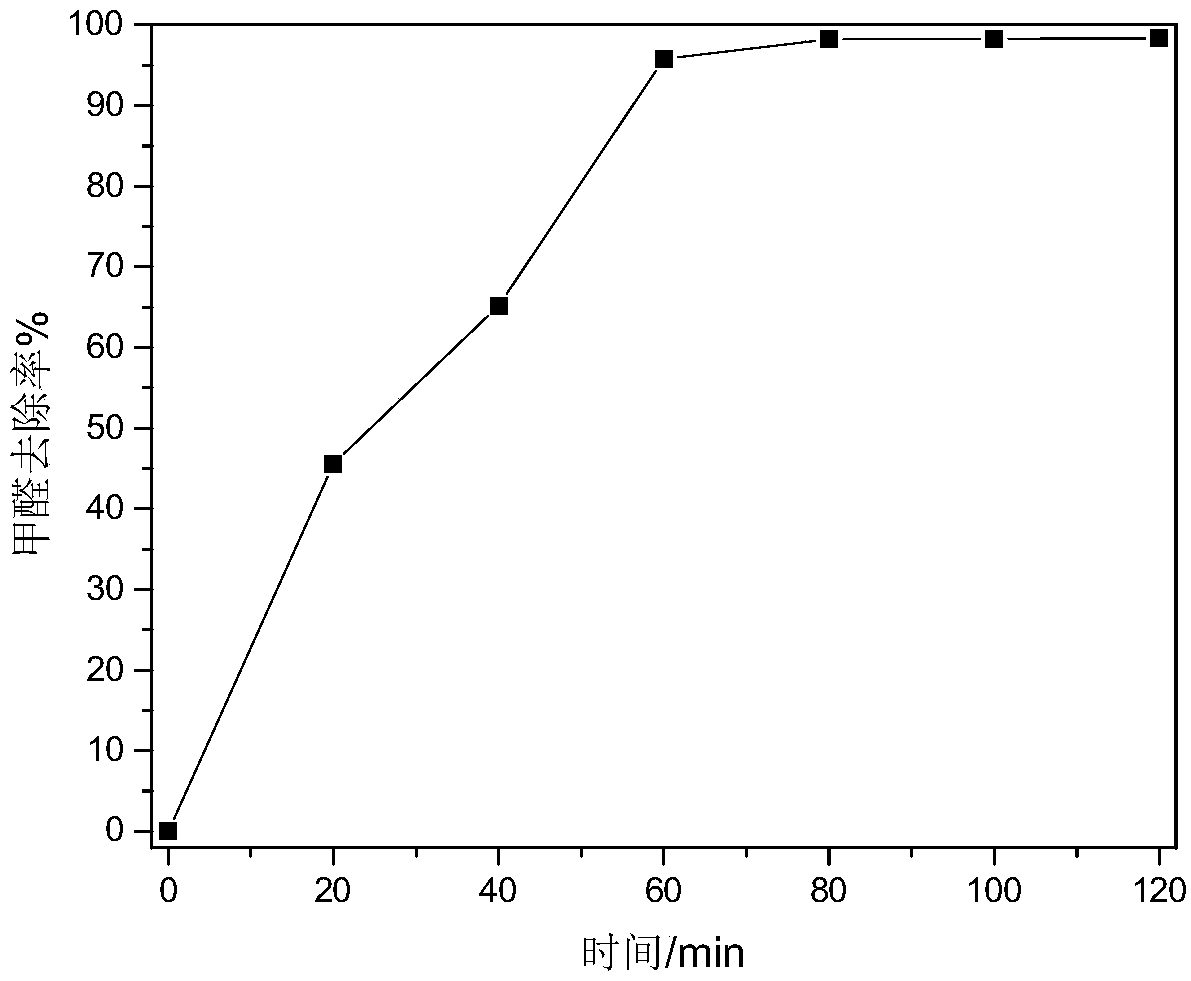
[0024] A remover for quickly removing indoor formaldehyde, the formaldehyde removal agent is configured according to the following weight ratio: 15 parts of sodium hypochlorite, 2 parts of dicobalt trioxide, 8 parts of polyacrylamide, 3 parts of potassium dihydrogen phosphate, 3 parts of ethanol, 5 parts of nano manganese dioxide, 64 parts of water. The pH value of the removal reagent is 10. After being sealed at 30°C for 1 hour, the removal reagent is sprayed, the formaldehyde concentration in the room before spraying is measured, and the concentration of formaldehyde is sampled at intervals of 20 minutes after spraying to obtain the formaldehyde concentration after spraying. Changes with time, such as figure 1 As shown, it can be seen that the removal rate of formaldehyde reaches more than 95% after 1 hour.
Embodiment 2
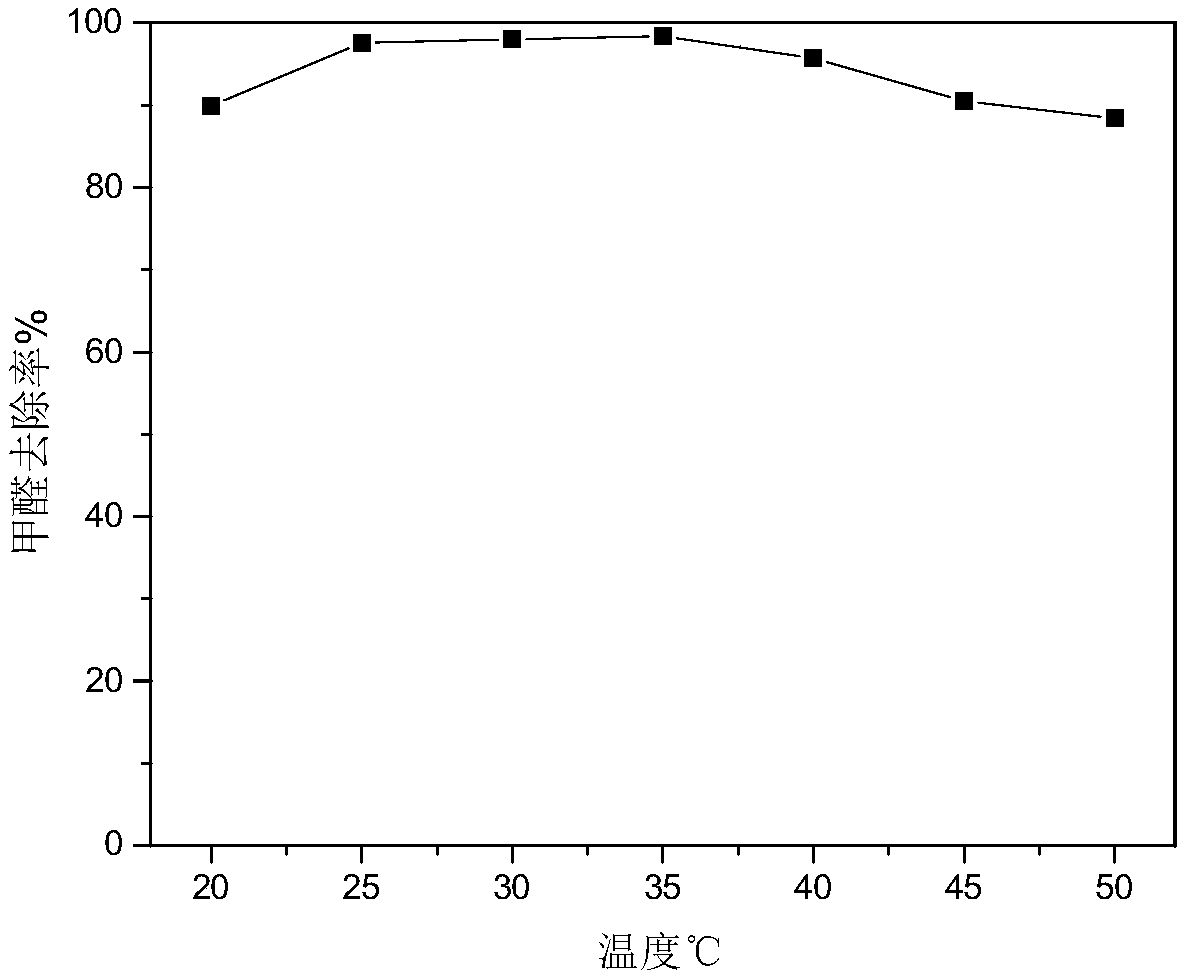
[0026] A remover for quickly removing indoor formaldehyde, the formaldehyde removal agent is configured according to the following weight ratio: 20 parts of sodium hypochlorite, 2 parts of dicobalt trioxide, 6 parts of polyacrylamide, 3 parts of potassium dihydrogen phosphate, 4 parts of ethanol, 8 parts of nano manganese dioxide, 57 parts of water. The pH value of the removal reagent is 10. Spray this removal reagent after sealing at different temperatures for 1 hour, measure the concentration of formaldehyde in the room before spraying and take samples to measure the concentration of formaldehyde 1 hour after spraying to obtain the change of formaldehyde concentration with the indoor temperature after spraying, as shown in figure 2 As shown, it can be seen that 25-35 ° C has a good removal effect on formaldehyde.
Embodiment 3
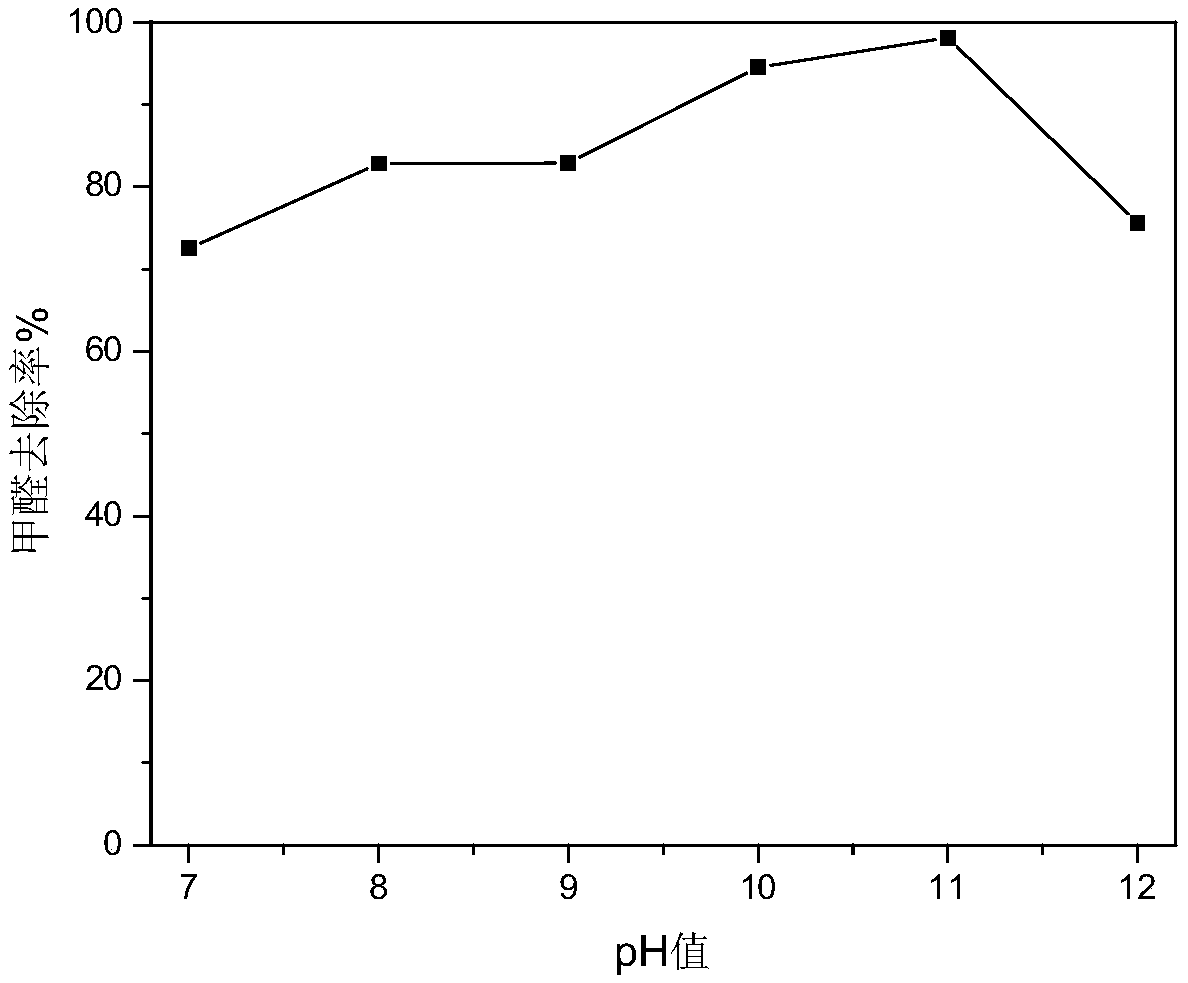
[0028] A remover for quickly removing indoor formaldehyde, the formaldehyde removal agent is configured according to the following weight ratio: 12 parts of sodium hypochlorite, 2 parts of dicobalt trioxide, 12 parts of polyacrylamide, 3 parts of potassium dihydrogen phosphate, 5 parts of ethanol, 6 parts of nano manganese dioxide, 60 parts of water. The pH of the removal reagent is set to 7-12. After being sealed at 35°C for 1 hour, the removal reagent is sprayed, the concentration of formaldehyde in the room before spraying is measured, and the concentration of formaldehyde is sampled and measured after spraying for 1 hour to obtain the change of formaldehyde concentration with pH after spraying, as shown in image 3 As shown, it can be seen that the removal effect of formaldehyde is good when the pH is 10-11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com