A kind of anti-human csf-1r monoclonal antibody and its application
A CSF-1R and monoclonal antibody technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, applications, etc., can solve problems that need further research and development, and achieve the effect of novel sequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
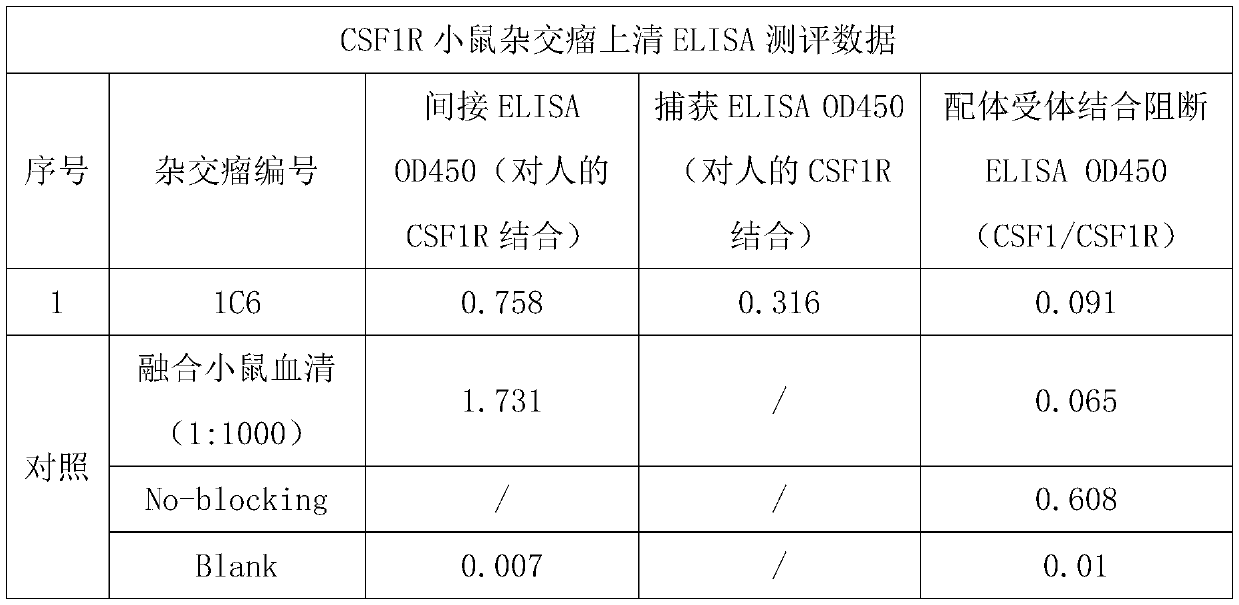
[0035] Example 1 Obtaining specific anti-CSF1R mouse monoclonal antibody by fusion hybridoma technology
[0036] 1.1 Animal Immunization
[0037] Mice were immunized according to general methods in the literature (E Harlow, D. Lane, Antibody: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1998). The immunogen was a recombinant human CSF1R (amino acid Ile20-Glu 512) protein (Acrobiosystems, Cat#CSR-H5258) containing a human IgG1 Fc-tag at the C-terminus. Recombinant human CSF1R his-tagged protein (Acro biosystems, cat#CSR-H5228) was used as the detection antigen for serum titer determination and hybridoma screening. Briefly, remove an appropriate amount of Freund's adjuvant into a 1.5ml EP tube and mix well with a shaker. Prepare antigen protein solution with PBS. Mix the adjuvant and the protein antigen solution according to the required amount, push each other through a syringe to fully emulsify the antigen to form a stable water-in-oil...
Embodiment 2
[0043] Example 2 In Vitro Assay for Determining the Functional Activity of CSF1R Monoclonal Antibodies
[0044] 2.1 Determination of antibody binding capacity based on capture ELSIA
[0045] A goat anti-mouse IgG Fcr-specific secondary antibody (Jackson Immuno Research, #115-006-071) was prepared with 1xPBS to a final concentration of 2μg / ml, and 100μl / well was added to a 96-well microtiter plate at 4°C Wrap overnight. The next day, after washing the plate 4 times with PBS containing 0.05% Tween 20 (ie 1×PBST), 200 μl / well of 5% nonfat dry milk in PBST was added and placed at 37 degrees for blocking for 2 hours. Wash the plate again, add 100 μl / well of diluted antibody solution or hybridoma supernatant, incubate at 37°C for 40 minutes, and then wash the plate 4 times. The prepared 60nM biotin-labeled human CSF1R Fc protein solution (in 2.5% nonfat dry milk in PBST) was added at 100 μl / well, incubated at 37°C for 40 minutes, and then the plate was washed 4 times. Add 1:10000...
Embodiment 3
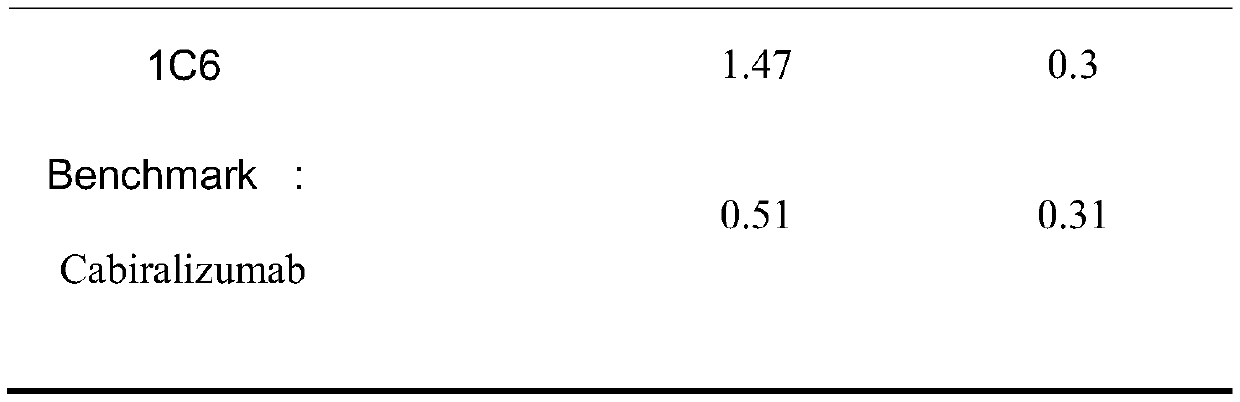
[0055] Example 3 Binding activity of anti-CSF1R mouse monoclonal antibody
[0056] According to the assay method described in Example 2, the binding activity of the CSF1R antibody was assessed and summarized in Table 2 below. Among them, Benchmark (Cabiralizumab) was used as a control, which is an existing uncommercialized anti-human CSF1R monoclonal antibody. It can be seen from Table 2 that the binding activity of the anti-human CSF-1R monoclonal antibody of the present invention to the human CSF1R antigen is significantly better than that of Benchmark.
[0057] Table 2 Binding activity of CSF1R antibodies
[0058]
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com