Novel form of sulfentrazone, process for its preparation and use thereof
A technology of sulfentrazone and methanesulfonamide, applied in the direction of botany equipment and methods, applications, herbicides and algicides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: Preparation of amorphous sulfentrazone according to the disclosure of US Patent No. 4,818,275 (Example 13, Example 14 and Example 15):
[0110] 1. 1-(5-Amino-2,4-dichlorophenyl)-3-methyl-4-difluoromethyl-Δ as an intermediate 2 -1, 2, 4 - three Preparation of oxazolin-5-one (embodiment 13)
[0111] Step A - Synthesis of pyruvate, 2,4-dichlorophenylhydrazone as intermediate
[0112] To a stirred solution of 16.2 g (0.07 mol) of commercially available 2,4-dichlorophenylhydrazine hydrochloride in 100 ml of ethanol was added 9.2 g (0.11 mol) of pyruvic acid in 100 ml of water in one portion. The reaction mixture was stirred for 10 minutes and the resulting solid was collected by filtration to give 13.5 g of pyruvic acid, 2,4-dichlorophenylhydrazone, m.p. 193°C-194°C when dry. This reaction was repeated several times.
[0113]
[0114] Step B - 1-(2,4-dichlorophenyl)-3-methyl-Δ as intermediate 2 -Synthesis of 1,2,4-triazolin-5-one
[0115] To a stir...
Embodiment 2
[0133] Example 2 - Crystallization from xylene
[0134] A sample of amorphous sulfentrazone (10 g) prepared as in Example 1 was placed in a three necked round bottom flask together with xylene (60 ml). The resulting slurry was heated to 90°C to obtain a homogeneous solution. The homogeneous solution was stirred at 90 °C for 2 h and insoluble particles (if any) were filtered and the solution was cooled slowly to 20-25 °C. After cooling, fine crystals formed and the resulting heterogeneous mixture was stirred at 20°C for 2 hours. Then, the slurry was filtered and washed with xylene (3ml) at 20°C. The filtered crystals were dried under vacuum at 60°C. The purity of the resulting crystalline product was about 98%, and the yield of recovered crystalline product was found to be about 85%.
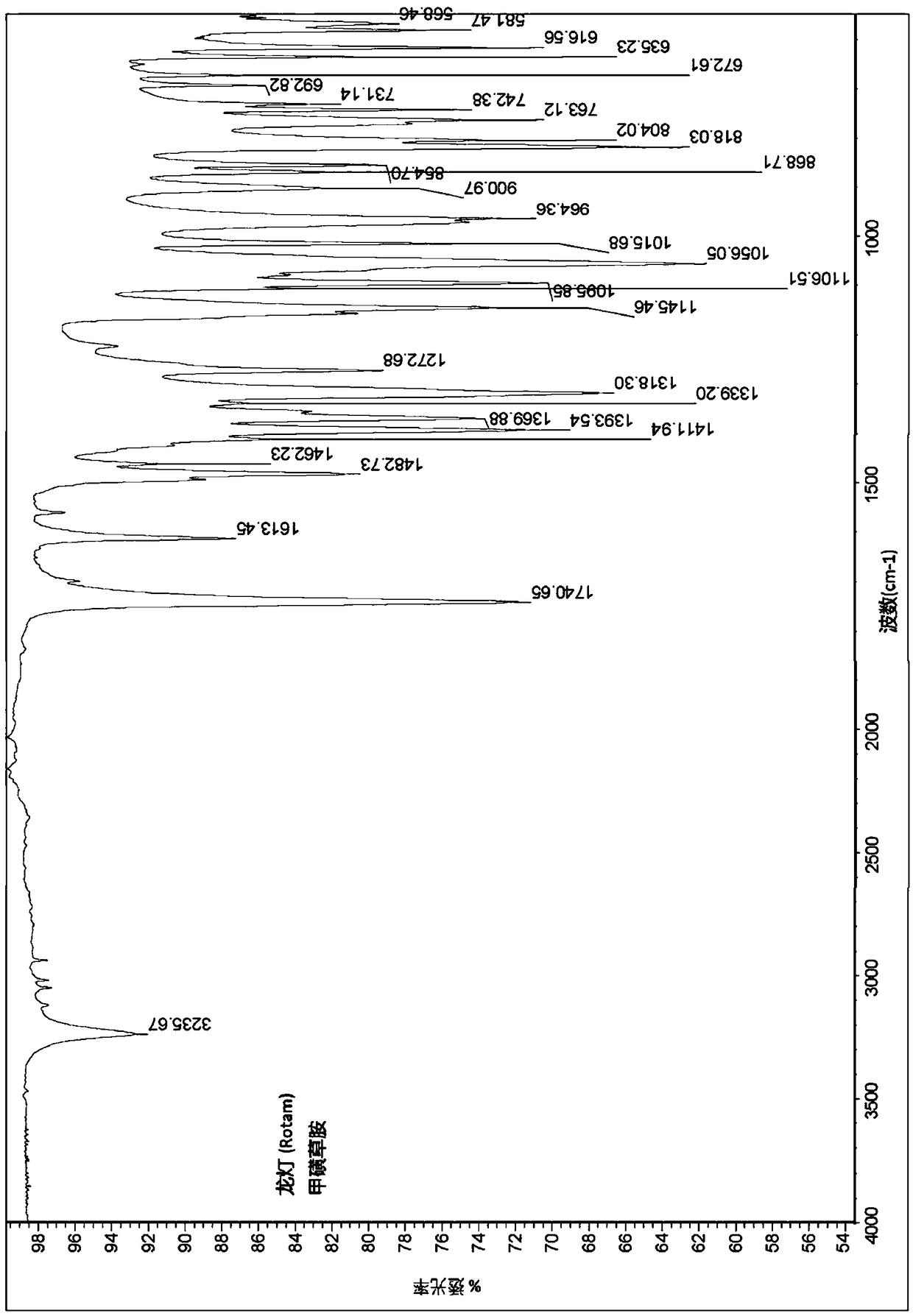
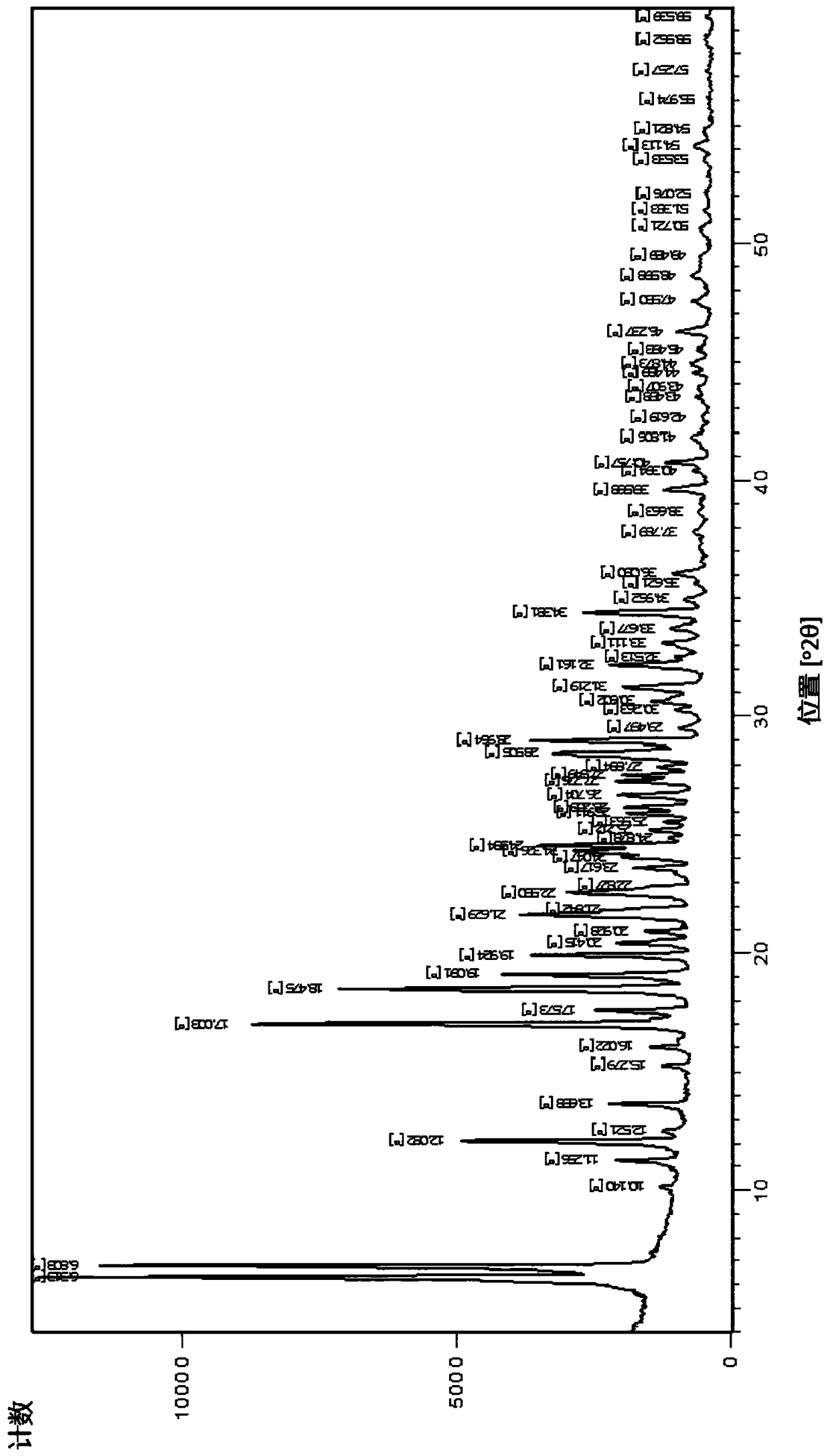
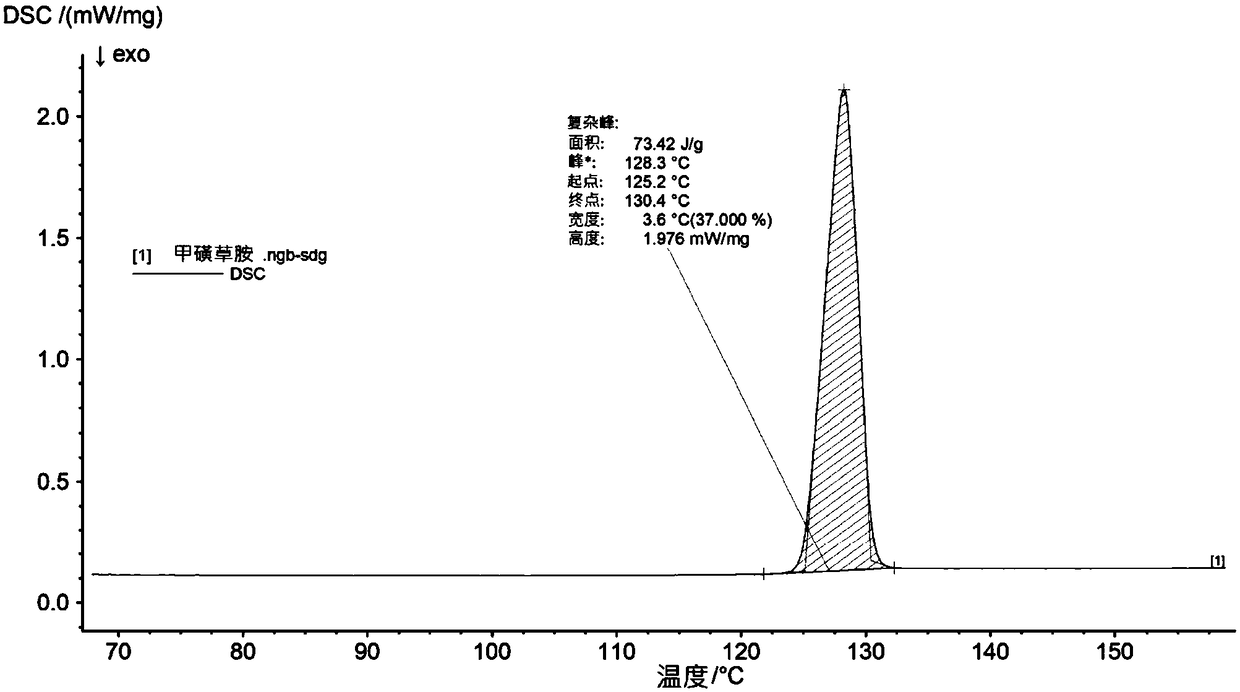
[0135] The obtained crystals were analyzed by IR spectroscopy, X-RPD and DSC and found to be as figure 1 , figure 2 and image 3 Sulfentrazone crystalline modification I shown.
[0136] ...
Embodiment 3
[0141] Example 3 - Crystallization from diethyl ketone
[0142] A sample of sulfentrazone (5 g) prepared as in Example 1 was placed in a three necked round bottom flask together with diethyl ketone (35 ml). The resulting slurry was heated to 80°C to obtain a homogeneous solution. The resulting hot solution was stirred at 80 °C for 2 h and insoluble particles (if any) were filtered and the solution was slowly cooled to 20 °C. After cooling, fine crystals formed and the resulting heterogeneous mixture was stirred at 20°C for 2 hours. Then, the slurry was filtered, washed with diethyl ketone (3 ml) at 20°C, and dried at 50°C under vacuum. The resulting crystalline product was about 98% pure and was found to be recovered in about 85% yield.
[0143] The crystals were characterized as crystalline modification I of sulfentrazone using IR spectroscopy, X-ray powder diffraction and DSC as described in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com