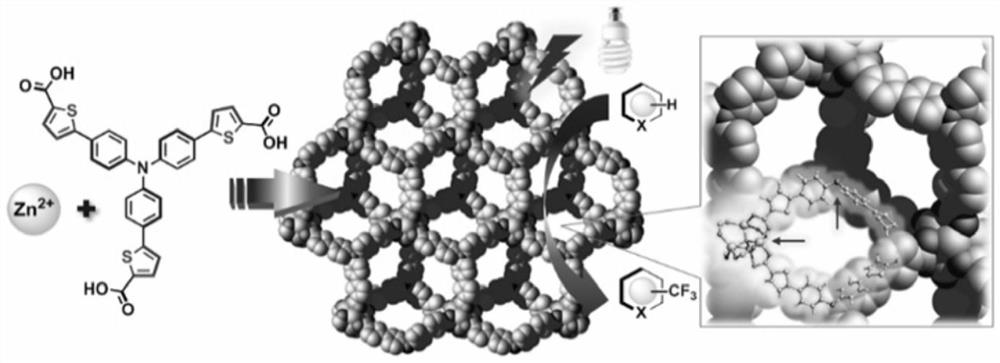
Preparation method and application of triphenylamine-based metal-organic complex with visible light-catalyzed trifluoromethylation performance of aromatic heterocyclic compounds
A technology for trifluoromethylation and aromatic heterocycles, which can be applied in the field of photocatalytic materials and can solve problems such as universal limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
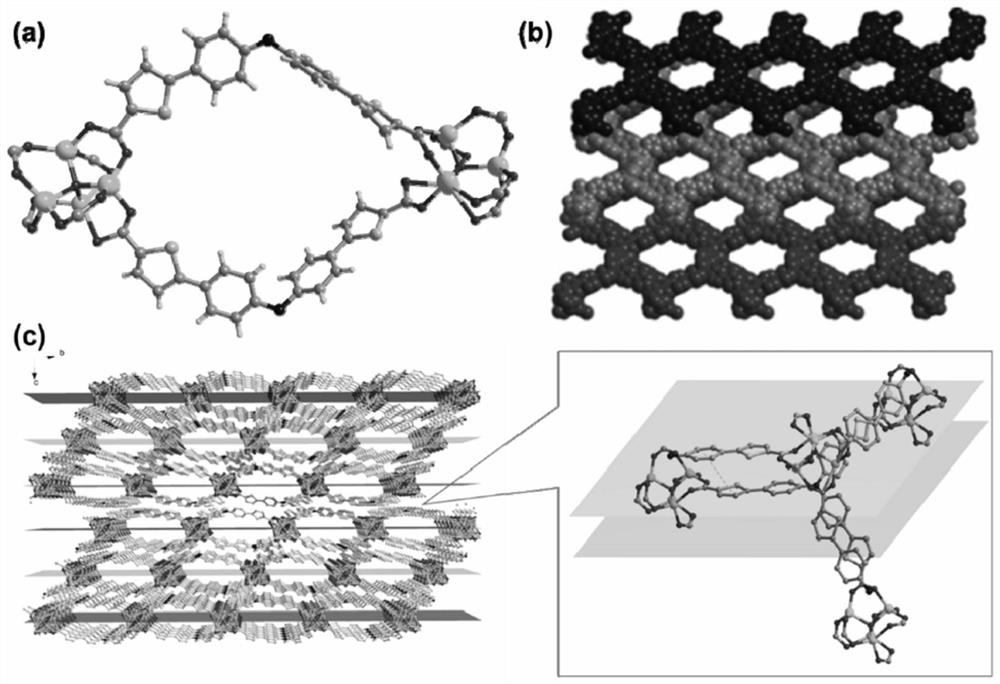
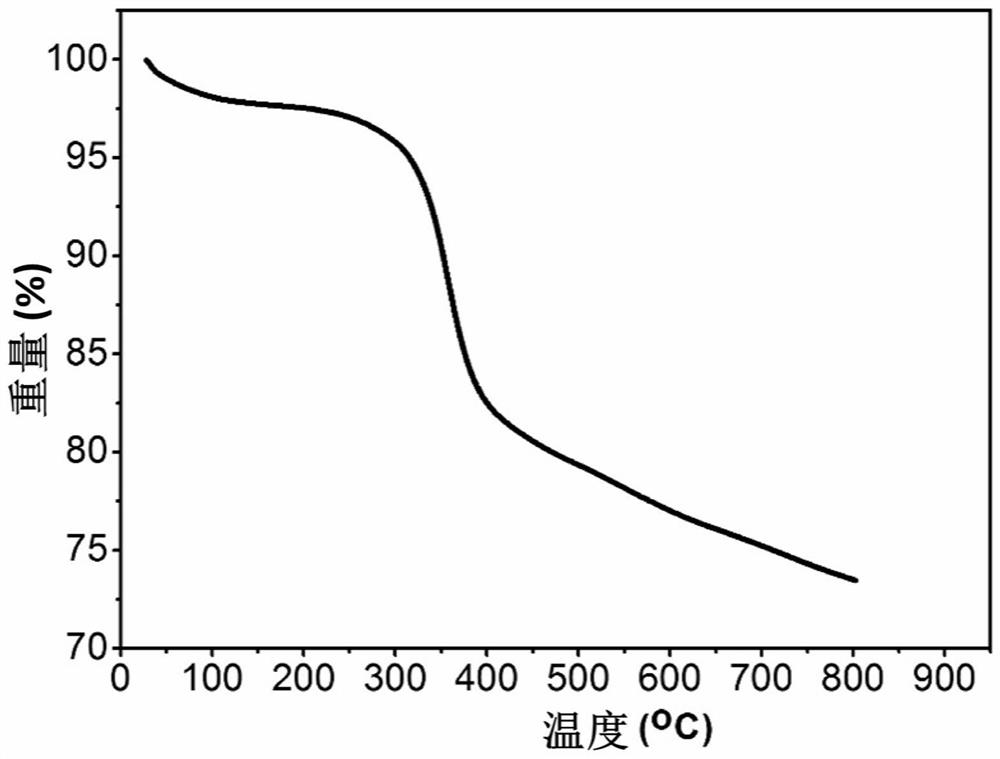
[0034] Tris[4-(5-hydroxyformyl-2-thienyl)phenyl]amine (93mg, 0.15mmol), Zn(NO 3 ) 2 ·6H 2 O (297mg, 1.0mmol) was dissolved in N,N'-dimethylformamide (DMF, 6mL) and stirred evenly, then it was taken out and placed in an oven, fired at 100°C for 72h, turned off the oven, cooled to room temperature, brownish red Rhombic bulk crystals are produced, filtered and dried to obtain the target material Zn-L with a yield of about 70%. Theoretical value of elemental analysis (%) (Zn 4 O)(C 33 h 18 NO 6 S 3 ) 2 : C 52.19, H 2.39, N 1.84, S, 12.67. Experimental values: C 52.02, H 2.51, N 1.96, S 12.48. The obtained target material structure is as figure 2 shown.
Embodiment 2
[0036] Tris[4-(5-hydroxyformyl-2-thienyl)phenyl]amine (93mg, 0.15mmol), Zn(NO 3 ) 2 ·6H 2 O (297mg, 1.0mmol) was dissolved in N,N'-dimethylformamide (DMF, 6mL) and stirred evenly, then the solution was placed in an oven, fired at 120°C for 72h, closed the oven, and cooled to room temperature , brown-red diamond-shaped bulk crystals were produced, filtered and dried to obtain the target material Zn-L with a yield of about 62%. Theoretical value of elemental analysis (%) (Zn 4 O)(C 33 h 18 NO 6 S 3 ) 2 : C 52.19, H 2.39, N 1.84, S, 12.67. Experimental values: C 52.06, H 2.49, N 1.93, S 12.52.
Embodiment 3
[0038] Tris[4-(5-hydroxyformyl-2-thienyl)phenyl]amine (93mg, 0.15mmol), Zn(NO 3 ) 2 ·6H 2 O (297mg, 1.0mmol) was dissolved in N,N'-dimethylformamide (DMF, 6mL) and stirred evenly, then the solution was placed in an oven, fired at 100°C for 90h, closed the oven, and cooled to room temperature , brown-red diamond-shaped bulk crystals were produced, filtered and dried to obtain the target material Zn-L with a yield of about 55%. Theoretical value of elemental analysis (%) (Zn 4 O)(C 33 h 18 NO 6 S 3 ) 2 : C 52.19, H 2.39, N 1.84, S, 12.67. Experimental values: C 52.09, H 2.45, N 1.94, S 12.45.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com